A novel B-domain O-glycoPEGylated FVIII (N8-GP) demonstrates full efficacy and prolonged effect in hemophilic mice models
- PMID: 23335368
- PMCID: PMC3596971
- DOI: 10.1182/blood-2012-01-407494
A novel B-domain O-glycoPEGylated FVIII (N8-GP) demonstrates full efficacy and prolonged effect in hemophilic mice models
Abstract
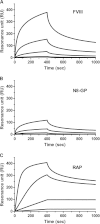
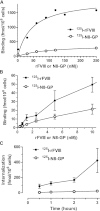
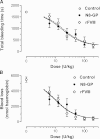
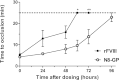
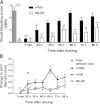
Frequent infusions of intravenous factor VIII (FVIII) are required to prevent bleeding associated with hemophilia A. To reduce the treatment burden, recombinant FVIII with a longer half-life was developed without changing the protein structure. FVIII-polyethylene glycol (PEG) conjugates were prepared using an enzymatic process coupling PEG (ranging from 10 to 80 kDa) selectively to a unique O-linked glycan in the FVIII B-domain. Binding to von Willebrand factor (VWF) was maintained for all conjugates. Upon cleavage by thrombin, the B-domain and the associated PEG were released, generating activated FVIII (FVIIIa) with the same primary structure and specific activity as native FVIIIa. In both FVIII- and VWF-deficient mice, the half-life was found to increase with the size of PEG. In vivo potency and efficacy of FVIII conjugated with a 40-kDa PEG (N8-GP) and unmodified FVIII were not different. N8-GP had a longer duration of effect in FVIII-deficient mouse models, approximately a twofold prolonged half-life in mice, rabbits, and cynomolgus monkeys; however, the prolongation was less pronounced in rats. Binding capacity of N8-GP on human monocyte-derived dendritic cells was reduced compared with unmodified FVIII, resulting in several-fold reduced cellular uptake. In conclusion, N8-GP has the potential to offer efficacious prevention and treatment of bleeds in hemophilia A at reduced dosing frequency.
Figures


Similar articles
-
Preclinical pharmacokinetics and biodistribution of subcutaneously administered glycoPEGylated recombinant factor VIII (N8-GP) and development of a human pharmacokinetic prediction model.J Thromb Haemost. 2018 Jun;16(6):1141-1152. doi: 10.1111/jth.14013. Epub 2018 May 10. J Thromb Haemost. 2018. PMID: 29582559
-
Prolonged effect of a new O-glycoPEGylated FVIII (N8-GP) in a murine saphenous vein bleeding model.Haemophilia. 2013 Nov;19(6):913-9. doi: 10.1111/hae.12198. Epub 2013 Jun 4. Haemophilia. 2013. PMID: 23730746
-
Enhancing the pharmacokinetic properties of recombinant factor VIII: first-in-human trial of glycoPEGylated recombinant factor VIII in patients with hemophilia A.J Thromb Haemost. 2013 Apr;11(4):670-8. doi: 10.1111/jth.12161. J Thromb Haemost. 2013. PMID: 23398640 Clinical Trial.
-
An overview of turoctocog alfa pegol (N8-GP; ESPEROCT® ) assay performance: Implications for postadministration monitoring.Haemophilia. 2020 Jan;26(1):156-163. doi: 10.1111/hae.13897. Epub 2019 Dec 6. Haemophilia. 2020. PMID: 31809565 Free PMC article. Review.
-
To serve and protect: The modulatory role of von Willebrand factor on factor VIII immunogenicity.Blood Rev. 2017 Sep;31(5):339-347. doi: 10.1016/j.blre.2017.07.001. Epub 2017 Jul 4. Blood Rev. 2017. PMID: 28716211 Review.
Cited by
-
Sensitive Measurement of Clinically Relevant Factor VIII Levels in Thrombin Generation Assays Requires Presence of Factor XIa.Thromb Haemost. 2023 Nov;123(11):1034-1041. doi: 10.1055/a-2101-7961. Epub 2023 May 26. Thromb Haemost. 2023. PMID: 37236229 Free PMC article.
-
The structural basis for the functional comparability of factor VIII and the long-acting variant recombinant factor VIII Fc fusion protein.J Thromb Haemost. 2017 Jun;15(6):1167-1179. doi: 10.1111/jth.13700. Epub 2017 May 3. J Thromb Haemost. 2017. PMID: 28397397 Free PMC article.
-
A field study evaluating the activity of N8-GP in spiked plasma samples at clinical haemostasis laboratories.Haemophilia. 2019 Sep;25(5):893-901. doi: 10.1111/hae.13813. Epub 2019 Jul 11. Haemophilia. 2019. PMID: 31294905 Free PMC article.
-
Current and emerging factor VIII replacement products for hemophilia A.Ther Adv Hematol. 2017 Oct;8(10):303-313. doi: 10.1177/2040620717721458. Epub 2017 Aug 26. Ther Adv Hematol. 2017. PMID: 29051801 Free PMC article. Review.
-
FVIII half-life extension by coadministration of a D'D3 albumin fusion protein in mice, rabbits, rats, and monkeys.Blood Adv. 2020 May 12;4(9):1870-1880. doi: 10.1182/bloodadvances.2019000999. Blood Adv. 2020. PMID: 32374879 Free PMC article.
References
-
- Hay CR. Prophylaxis in adults with haemophilia. Haemophilia. 2007;13(suppl 2):10–15. - PubMed
-
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. - PubMed
-
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. - PubMed
-
- Bailon P, Won CY. PEG-modified biopharmaceuticals. Expert Opin Drug Deliv. 2009;6(1):1–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

