Suppression of inflammatory responses during myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis is regulated by AKT3 signaling
- PMID: 23335751
- PMCID: PMC3564598
- DOI: 10.4049/jimmunol.1201387
Suppression of inflammatory responses during myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis is regulated by AKT3 signaling
Abstract
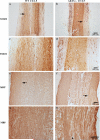
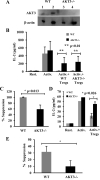
AKT3, a member of the serine/threonine kinase AKT family, is involved in a variety of biologic processes. AKT3 is expressed in immune cells and is the major AKT isoform in the CNS representing 30% of the total AKT expressed in spinal cord, and 50% in the brain. Myelin-oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE) is a mouse model in which lymphocytes and monocytes enter the CNS, resulting in inflammation, demyelination, and axonal injury. We hypothesized that during EAE, deletion of AKT3 would negatively affect the CNS of AKT3(-/-) mice, making them more susceptible to CNS damage. During acute EAE, AKT3(-/-)mice were more severely affected than wild type (WT) mice. Evaluation of spinal cords showed that during acute and chronic disease, AKT3(-/-) spinal cords had more demyelination compared with WT spinal cords. Quantitative RT-PCR determined higher levels of IL-2, IL-17, and IFN-γ mRNA in spinal cords from AKT3(-/-) mice than WT. Experiments using bone marrow chimeras demonstrated that AKT3(-/-) mice receiving AKT3-deficient bone marrow cells had elevated clinical scores relative to control WT mice reconstituted with WT cells, indicating that altered function of both CNS cells and bone marrow-derived immune cells contributed to the phenotype. Immunohistochemical analysis revealed decreased numbers of Foxp3(+) regulatory T cells in the spinal cord of AKT3(-/-) mice compared with WT mice, whereas in vitro suppression assays showed that AKT3-deficient Th cells were less susceptible to regulatory T cell-mediated suppression than their WT counterparts. These results indicate that AKT3 signaling contributes to the protection of mice against EAE.
Figures







References
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. - PubMed
-
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI063537/AI/NIAID NIH HHS/United States
- R21 AI079363/AI/NIAID NIH HHS/United States
- R21 NS079144/NS/NINDS NIH HHS/United States
- AI059738/AI/NIAID NIH HHS/United States
- T32NS007098/NS/NINDS NIH HHS/United States
- T32 GM007491/GM/NIGMS NIH HHS/United States
- R21 NS061128/NS/NINDS NIH HHS/United States
- R56 AI059738/AI/NIAID NIH HHS/United States
- GM007288/GM/NIGMS NIH HHS/United States
- R01 NS038102/NS/NINDS NIH HHS/United States
- R01 AI059738/AI/NIAID NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- T32 NS007098/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

