Lateral mobility of L-type calcium channels in synaptic terminals of retinal bipolar cells
- PMID: 23335847
- PMCID: PMC3548577
Lateral mobility of L-type calcium channels in synaptic terminals of retinal bipolar cells
Abstract
Purpose: Efficient and precise release of glutamate from retinal bipolar cells is ensured by the positioning of L-type Ca(2+) channels close to release sites at the base of the synaptic ribbon. We investigated whether Ca(2+) channels at bipolar cell ribbon synapses are fixed in position or capable of moving in the membrane.
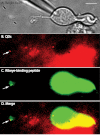
Methods: We tracked the movements of individual L-type Ca(2+) channels in bipolar cell terminals after labeling channels with quantum dots (QDs) attached to α(2)δ(4) accessory Ca(2+) channel subunits via intermediary antibodies.
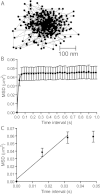
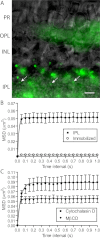
Results: We found that individual Ca(2+) channels moved within a confined domain of 0.13-0.15 μm(2) in bipolar cell terminals, similar to ultrastructural estimates of the surface area of the active zone beneath the ribbon. Disruption of actin expanded the confinement domain indicating that cytoskeletal interactions help to confine channels at the synapse, but the relatively large diffusion coefficients of 0.3-0.45 μm(2)/s suggest that channels are not directly anchored to actin. Unlike photoreceptor synapses, removing membrane cholesterol did not change domain size, indicating that lipid rafts are not required to confine Ca(2+) channels at bipolar cell ribbon synapses.
Conclusions: The ability of Ca(2+) channels to move within the presynaptic active zone suggests that regulating channel mobility may affect release from bipolar cell terminals.
Figures

References
-
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
