Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition
- PMID: 23335884
- PMCID: PMC3541516
- DOI: 10.3389/fncir.2012.00116
Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition
Abstract
Following the fundamental recognition of its involvement in sensory-motor coordination and learning, the cerebellum is now also believed to take part in the processing of cognition and emotion. This hypothesis is recurrent in numerous papers reporting anatomical and functional observations, and it requires an explanation. We argue that a similar circuit structure in all cerebellar areas may carry out various operations using a common computational scheme. On the basis of a broad review of anatomical data, it is conceivable that the different roles of the cerebellum lie in the specific connectivity of the cerebellar modules, with motor, cognitive, and emotional functions (at least partially) segregated into different cerebro-cerebellar loops. We here develop a conceptual and operational framework based on multiple interconnected levels (a meta-levels hypothesis): from cellular/molecular to network mechanisms leading to generation of computational primitives, thence to high-level cognitive/emotional processing, and finally to the sphere of mental function and dysfunction. The main concept explored is that of intimate interplay between timing and learning (reminiscent of the "timing and learning machine" capabilities long attributed to the cerebellum), which reverberates from cellular to circuit mechanisms. Subsequently, integration within large-scale brain loops could generate the disparate cognitive/emotional and mental functions in which the cerebellum has been implicated. We propose, therefore, that the cerebellum operates as a general-purpose co-processor, whose effects depend on the specific brain centers to which individual modules are connected. Abnormal functioning in these loops could eventually contribute to the pathogenesis of major brain pathologies including not just ataxia but also dyslexia, autism, schizophrenia, and depression.
Keywords: autism; cerebellum; cognition; dyslexia; motor control; prediction; schizophrenia; timing.
Figures
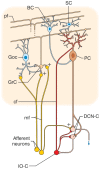
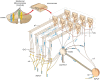
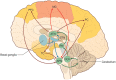
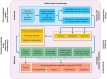
References
-
- Albus J. S. (1972). A theory of cerebellar function. Math. Biosci. 10, 25–61
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

