Sub-millisecond closed-loop feedback stimulation between arbitrary sets of individual neurons
- PMID: 23335887
- PMCID: PMC3541546
- DOI: 10.3389/fncir.2012.00121
Sub-millisecond closed-loop feedback stimulation between arbitrary sets of individual neurons
Abstract
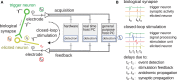
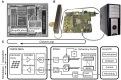
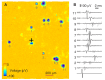
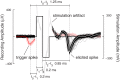
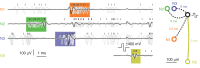
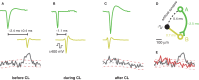
We present a system to artificially correlate the spike timing between sets of arbitrary neurons that were interfaced to a complementary metal-oxide-semiconductor (CMOS) high-density microelectrode array (MEA). The system features a novel reprogrammable and flexible event engine unit to detect arbitrary spatio-temporal patterns of recorded action potentials and is capable of delivering sub-millisecond closed-loop feedback of electrical stimulation upon trigger events in real-time. The relative timing between action potentials of individual neurons as well as the temporal pattern among multiple neurons, or neuronal assemblies, is considered an important factor governing memory and learning in the brain. Artificially changing timings between arbitrary sets of spiking neurons with our system could provide a "knob" to tune information processing in the network.
Keywords: LTD; STDP; acausal stimulation; closed-loop; high-density microelectrode array; sub-millisecond.
Figures


References
-
- Abeles M., Gerstein G. L. (1988). Detecting spatiotemporal firing patterns among simultaneously recorded single neurons. J. Neurophysiol. 60, 909–924 - PubMed
-
- Azin M., Guggenmos D. J., Barbay S., Nudo R. J., Mohseni P. (2011). A battery-powered activity-dependent intracortical microstimulation ic for brain-machine-brain interface. IEEE J. Solid-State Circ. 46, 731–745
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

