HSV, axonal transport and Alzheimer's disease: in vitro and in vivo evidence for causal relationships
- PMID: 23335944
- PMCID: PMC3546524
- DOI: 10.2217/fvl.12.81
HSV, axonal transport and Alzheimer's disease: in vitro and in vivo evidence for causal relationships
Abstract
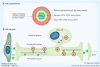
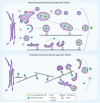
HSV, a neurotropic virus, travels within neuronal processes by fast axonal transport. During neuronal infection HSV travels retrograde from the sensory nerve terminus to the neuronal cell body, where it replicates or enters latency. During replication HSV travels anterograde from the cell body to the nerve terminus. Postmortem studies find a high frequency of HSV DNA in the trigeminal ganglia as well as the brain. Studies correlating HSV with Alzheimer's disease (AD) have been controversial. Here we review clinical evidence supporting such a link. Furthermore, the author describes experimental data showing physical interactions between nascent HSV particles and host transport machinery implicated in AD. The author concludes that the complexity of this relationship has been insufficiently explored, although the relative ease and nontoxicity of a potential anti-HSV treatment for AD demands further study.
Figures

References
-
- Roizman B, Knipe D. Herpes simplex viruses and their replication. In: Knipe D, Howley P, editors. Fields Virology. Lippincott Williams & Wilkins; PA, USA: 2001. pp. 2399–2461.
-
- Su YH, Moxley MJ, Ng AK, et al. Stability and circularization of herpes simplex virus type 1 genomes in quiescently infected PC12 cultures. J. Gen. Virol. 2002;83(Pt 12):2943–2950. - PubMed
-
- Mellerick DM, Fraser NW. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987;158(2):265–275. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
