Aminoglycosylation can enhance the G-quadruplex binding activity of epigallocatechin
- PMID: 23335983
- PMCID: PMC3545880
- DOI: 10.1371/journal.pone.0053962
Aminoglycosylation can enhance the G-quadruplex binding activity of epigallocatechin
Abstract
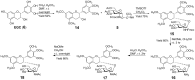
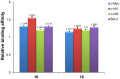
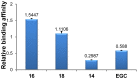
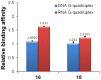
With the aim of enhancing G-quadruplex binding activity, two new glucosaminosides (16, 18) of penta-methylated epigallocatechin were synthesized by chemical glycosylation. Subsequent ESI-TOF-MS analysis demonstrated that these two glucosaminoside derivatives exhibit much stronger binding activity to human telomeric DNA and RNA G-quadruplexes than their parent structure (i.e., methylated EGC) (14) as well as natural epigallocatechin (EGC, 6). The DNA G-quadruplex binding activity of 16 and 18 is even more potent than strong G-quadruplex binder quercetin, which has a more planar structure. These two synthetic compounds also showed a higher binding strength to human telomeric RNA G-quadruplex than its DNA counterpart. Analysis of the structure-activity relationship revealed that the more basic compound, 16, has a higher binding capacity with DNA and RNA G-quadruplexes than its N-acetyl derivative, 18, suggesting the importance of the basicity of the aminoglycoside for G-quadruplex binding activity. Molecular docking simulation predicted that the aromatic ring of 16 π-stacks with the aromatic ring of guanine nucleotides, with the glucosamine moiety residing in the groove of G-quadruplex. This research indicates that glycosylation of natural products with aminosugar can significantly enhance their G-quadruplex binding activities, thus is an effective way to generate small molecules targeting G-quadruplexes in nucleic acids. In addition, this is the first report that green tea catechin can bind to nucleic acid G-quadruplex structures.
Conflict of interest statement
Figures




References
-
- Lipps HJ, Rhodes D (2009) G-quadruplex structures: in vivo evidence and function. Trends Cell Biol 19: 414–422. - PubMed
-
- Neidle S, Parkinson GN (2003) The structure of telomeric DNA. Curr Opin Struct Biol 13: 275–283. - PubMed
-
- Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J (2007) Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318: 798–801. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

