Live cell imaging techniques to study T cell trafficking across the blood-brain barrier in vitro and in vivo
- PMID: 23336847
- PMCID: PMC3560242
- DOI: 10.1186/2045-8118-10-7
Live cell imaging techniques to study T cell trafficking across the blood-brain barrier in vitro and in vivo
Abstract
Background: The central nervous system (CNS) is an immunologically privileged site to which access for circulating immune cells is tightly controlled by the endothelial blood-brain barrier (BBB) located in CNS microvessels. Under physiological conditions immune cell migration across the BBB is low. However, in neuroinflammatory diseases such as multiple sclerosis, many immune cells can cross the BBB and cause neurological symptoms. Extravasation of circulating immune cells is a multi-step process that is regulated by the sequential interaction of different adhesion and signaling molecules on the immune cells and on the endothelium. The specialized barrier characteristics of the BBB, therefore, imply the existence of unique mechanisms for immune cell migration across the BBB.
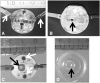
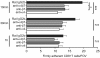
Methods and design: An in vitro mouse BBB model maintaining physiological barrier characteristics in a flow chamber and combined with high magnification live cell imaging, has been established. This model enables the molecular mechanisms involved in the multi-step extravasation of T cells across the in vitro BBB, to be defined with high-throughput analyses. Subsequently these mechanisms have been verified in vivo using a limited number of experimental animals and a spinal cord window surgical technique. The window enables live observation of the dynamic interaction between T cells and spinal cord microvessels under physiological and pathological conditions using real time epifluorescence intravital imaging. These in vitro and in vivo live cell imaging methods have shown that the BBB endothelium possesses unique and specialized mechanisms involved in the multi-step T cell migration across this endothelial barrier under physiological flow. The initial T cell interaction with the endothelium is either mediated by T cell capture or by T cell rolling. Arrest follows, and then T cells polarize and especially CD4+ T cells crawl over long distances against the direction of flow to find the rare sites permissive for diapedesis through the endothelium.
Discussion: The sequential use of in vitro and in vivo live cell imaging of T cells interacting with the BBB allows us to delineate the kinetics and molecular determinants involved in multistep extravasation of encephalitogenic T cells across the BBB.
Figures
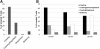

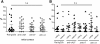
References
-
- Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood–brain barrier endothelium. J Immunol. 2010;185:4846–4855. doi: 10.4049/jimmunol.0903732. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

