Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells
- PMID: 23337808
- PMCID: PMC3570108
- DOI: 10.1084/jem.20120363
Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells
Abstract
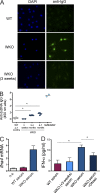
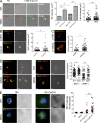
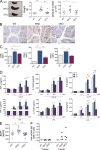
Mutations in Wiskott-Aldrich syndrome (WAS) protein (WASp), a regulator of actin dynamics in hematopoietic cells, cause WAS, an X-linked primary immunodeficiency characterized by recurrent infections and a marked predisposition to develop autoimmune disorders. The mechanisms that link actin alterations to the autoimmune phenotype are still poorly understood. We show that chronic activation of plasmacytoid dendritic cells (pDCs) and elevated type-I interferon (IFN) levels play a role in WAS autoimmunity. WAS patients display increased expression of type-I IFN genes and their inducible targets, alteration in pDCs numbers, and hyperresponsiveness to TLR9. Importantly, ablating IFN-I signaling in WASp null mice rescued chronic activation of conventional DCs, splenomegaly, and colitis. Using WASp-deficient mice, we demonstrated that WASp null pDCs are intrinsically more responsive to multimeric agonist of TLR9 and constitutively secrete type-I IFN but become progressively tolerant to further stimulation. By acute silencing of WASp and actin inhibitors, we show that WASp-mediated actin polymerization controls intracellular trafficking and compartmentalization of TLR9 ligands in pDCs restraining exaggerated activation of the TLR9-IFN-α pathway. Together, these data highlight the role of actin dynamics in pDC innate functions and imply the pDC-IFN-α axis as a player in the onset of autoimmune phenomena in WAS disease.
Figures






References
-
- Agrawal H., Jacob N., Carreras E., Bajana S., Putterman C., Turner S., Neas B., Mathian A., Koss M.N., Stohl W., et al. 2009. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J. Immunol. 183:6021–6029 10.4049/jimmunol.0803872 - DOI - PMC - PubMed
-
- Andreansky S., Liu H., Turner S., McCullers J.A., Lang R., Rutschman R., Doherty P.C., Murray P.J., Nienhuis A.W., Strom T.S. 2005. WASP- mice exhibit defective immune responses to influenza A virus, Streptococcus pneumoniae, and Mycobacterium bovis BCG. Exp. Hematol. 33:443–451 10.1016/j.exphem.2004.12.006 - DOI - PubMed
-
- Badour K., Zhang J., Shi F., McGavin M.K., Rampersad V., Hardy L.A., Field D., Siminovitch K.A. 2003. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 18:141–154 10.1016/S1074-7613(02)00516-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

