Sorafenib induces autophagy and suppresses activation of human macrophage
- PMID: 23337882
- PMCID: PMC7106104
- DOI: 10.1016/j.intimp.2013.01.006
Sorafenib induces autophagy and suppresses activation of human macrophage
Erratum in
- Int Immunopharmacol. 2013 May;16(1):123. Liu, Tsan-Pai [corrected to Liu, Tsang-Pai]
Abstract
Background: Sorafenib, a multi-kinase inhibitor approved for treatment of advanced renal cell carcinoma and other malignancies, has been shown as a modulator for dendritic cells. This study was designed to examine the effects of sorafenib on macrophages, the major ontogeny of innate immunity.
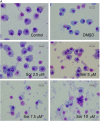
Materials and methods: Macrophages were derived from sorted CD14(+) monocytes of human peripheral blood mononuclear cells. Cell viability and surface antigens were examined by trypan blue analysis. Autophagy was characterized by light microscopy and transmission electron microscopy for morphology, Western blotting for microtubule associated light chain protein 3B (LC-3B) I lipidation, and acridine orange staining for acidic component vacuoles. Soluble factors contained in culture medium and serum were measured by ELISA.
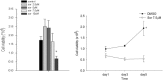
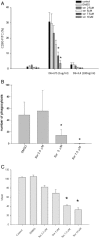
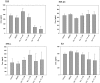
Results: We found that sorafenib inhibited the viability of macrophages accompanied by morphological changes characteristic of autophagy. This autophagy-inducing effect was validated by LC3B-I lipidation and autophagosome accumulation. The surface antigen expression and the function of activated macrophages were inhibited by sorafenib, including the expression of co-stimulatory molecule CD80, phagocytosis, and the production of reactive oxygen species. The secretion of IL-10, but not IL-6, TNF-α nor TGF-β, was reduced by sorafenib.
Conclusion: Sorafenib, in addition to being a cancer targeted therapeutic agent, can induce autophagy and modulate the function of human macrophages.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures



References
-
- Wilhelm S.M., Carter C., Tang L., Wilkie D., McNabola A., Rong H. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. - PubMed
-
- Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. - PubMed
-
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Hsieh C.H., Jeng K.S., Lin C.C., Chen C.K., Liu C.Y., Lin C.P. Combination of sorafenib and intensity modulated radiotherapy for unresectable hepatocellular carcinoma. Clin Drug Investig. 2009;29:65–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

