Taste responses in mice lacking taste receptor subunit T1R1
- PMID: 23339178
- PMCID: PMC3624863
- DOI: 10.1113/jphysiol.2012.236604
Taste responses in mice lacking taste receptor subunit T1R1
Abstract
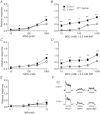
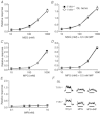
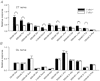
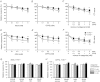
The T1R1 receptor subunit acts as an umami taste receptor in combination with its partner, T1R3. In addition, metabotropic glutamate receptors (brain and taste variants of mGluR1 and mGluR4) are thought to function as umami taste receptors. To elucidate the function of T1R1 and the contribution of mGluRs to umami taste detection in vivo, we used newly developed knock-out (T1R1(-/-)) mice, which lack the entire coding region of the Tas1r1 gene and express mCherry in T1R1-expressing cells. Gustatory nerve recordings demonstrated that T1R1(-/-) mice exhibited a serious deficit in inosine monophosphate-elicited synergy but substantial residual responses to glutamate alone in both chorda tympani and glossopharyngeal nerves. Interestingly, chorda tympani nerve responses to sweeteners were smaller in T1R1(-/-) mice. Taste cell recordings demonstrated that many mCherry-expressing taste cells in T1R1(+/-) mice responded to sweet and umami compounds, whereas those in T1R1(-/-) mice responded to sweet stimuli. The proportion of sweet-responsive cells was smaller in T1R1(-/-) than in T1R1(+/-) mice. Single-cell RT-PCR demonstrated that some single mCherry-expressing cells expressed all three T1R subunits. Chorda tympani and glossopharyngeal nerve responses to glutamate were significantly inhibited by addition of mGluR antagonists in both T1R1(-/-) and T1R1(+/-) mice. Conditioned taste aversion tests demonstrated that both T1R1(-/-) and T1R1(+/-) mice were equally capable of discriminating glutamate from other basic taste stimuli. Avoidance conditioned to glutamate was significantly reduced by addition of mGluR antagonists. These results suggest that T1R1-expressing cells mainly contribute to umami taste synergism and partly to sweet sensitivity and that mGluRs are involved in the detection of umami compounds.
Figures






Comment in
-
Sweet umami: the twain shall meet.J Physiol. 2013 Apr 1;591(7):1597. doi: 10.1113/jphysiol.2013.251611. J Physiol. 2013. PMID: 23547193 Free PMC article. No abstract available.
Similar articles
-
Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice.J Physiol. 2015 Feb 15;593(4):1021-34. doi: 10.1113/jphysiol.2014.284703. Epub 2015 Jan 22. J Physiol. 2015. PMID: 25529865 Free PMC article.
-
Multiple receptors underlie glutamate taste responses in mice.Am J Clin Nutr. 2009 Sep;90(3):747S-752S. doi: 10.3945/ajcn.2009.27462J. Epub 2009 Jul 1. Am J Clin Nutr. 2009. PMID: 19571210
-
Detection of sweet and umami taste in the absence of taste receptor T1r3.Science. 2003 Aug 8;301(5634):850-3. doi: 10.1126/science.1087155. Epub 2003 Jul 17. Science. 2003. PMID: 12869700
-
Multiple receptor systems for glutamate detection in the taste organ.Biol Pharm Bull. 2008 Oct;31(10):1833-7. doi: 10.1248/bpb.31.1833. Biol Pharm Bull. 2008. PMID: 18827337 Review.
-
Taste information derived from T1R-expressing taste cells in mice.Biochem J. 2016 Mar 1;473(5):525-36. doi: 10.1042/BJ20151015. Biochem J. 2016. PMID: 26912569 Review.
Cited by
-
Sweet umami: the twain shall meet.J Physiol. 2013 Apr 1;591(7):1597. doi: 10.1113/jphysiol.2013.251611. J Physiol. 2013. PMID: 23547193 Free PMC article. No abstract available.
-
Glutamate: Tastant and Neuromodulator in Taste Buds.Adv Nutr. 2016 Jul 15;7(4):823S-7S. doi: 10.3945/an.115.011304. Print 2016 Jul. Adv Nutr. 2016. PMID: 27422519 Free PMC article. Review.
-
TRPs in taste and chemesthesis.Handb Exp Pharmacol. 2014;223:827-71. doi: 10.1007/978-3-319-05161-1_5. Handb Exp Pharmacol. 2014. PMID: 24961971 Free PMC article. Review.
-
Amiloride-Insensitive Salt Taste Is Mediated by Two Populations of Type III Taste Cells with Distinct Transduction Mechanisms.J Neurosci. 2016 Feb 10;36(6):1942-53. doi: 10.1523/JNEUROSCI.2947-15.2016. J Neurosci. 2016. PMID: 26865617 Free PMC article.
-
Diet Preference, Feed Efficiency and Expression of the Sodium-Dependent Glucose Transporter Isoform 1 and Sweet Taste Receptors in the Jejunum of Lambs Supplemented with Different Flavours.Animals (Basel). 2023 Apr 20;13(8):1417. doi: 10.3390/ani13081417. Animals (Basel). 2023. PMID: 37106980 Free PMC article.
References
-
- Behrens M, Meyerhof W, Hellfritsch C, Hofmann T. Sweet and umami taste: natural products, their chemosensory targets, and beyond. Angew Chem Int Ed Engl. 2011;50:2220–2242. - PubMed
-
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nature Neurosci. 2000;3:113–119. - PubMed
-
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Pérez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. - PubMed
-
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1R3. Science. 2003;301:850–853. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

