The dynamic relationship between polyandry and selfish genetic elements
- PMID: 23339240
- PMCID: PMC3576583
- DOI: 10.1098/rstb.2012.0049
The dynamic relationship between polyandry and selfish genetic elements
Abstract
Selfish genetic elements (SGEs) are ubiquitous in eukaryotes and bacteria, and make up a large part of the genome. They frequently target sperm to increase their transmission success, but these manipulations are often associated with reduced male fertility. Low fertility of SGE-carrying males is suggested to promote polyandry as a female strategy to bias paternity against male carriers. Support for this hypothesis is found in several taxa, where SGE-carrying males have reduced sperm competitive ability. In contrast, when SGEs give rise to reproductive incompatibilities between SGE-carrying males and females, polyandry is not necessarily favoured, irrespective of the detrimental impact on male fertility. This is due to the frequency-dependent nature of these incompatibilities, because they will decrease in the population as the frequency of SGEs increases. However, reduced fertility of SGE-carrying males can prevent the successful population invasion of SGEs. In addition, SGEs can directly influence male and female mating behaviour, mating rates and reproductive traits (e.g. female reproductive tract length and male sperm). This reveals a potent and dynamic interaction between SGEs and polyandry highlighting the potential to generate sexual selection and conflict, but also indicates that polyandry can promote harmony within the genome by undermining the spread of SGEs.
Figures
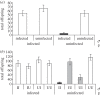
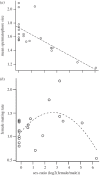
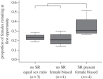
References
-
- Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. London, UK: Academic Press
-
- Birkhead TR, Hosken DJ, Pitnick S. 2009. Sperm biology: an evolutionary perspective. Burlington, MA: Academic Press
-
- Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) - DOI - DOI
-
- Crudgington HS, Siva-Jothy MT. 2000. Genital damage, kicking and early death. Nature 407, 855–856 10.1038/35038154 (doi:10.1038/35038154) - DOI - DOI - PubMed
-
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 10.1038/373241a0 (doi:10.1038/373241a0) - DOI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
