Phenylalanine hydroxylase misfolding and pharmacological chaperones
- PMID: 23339306
- PMCID: PMC3664513
- DOI: 10.2174/1568026611212220008
Phenylalanine hydroxylase misfolding and pharmacological chaperones
Abstract
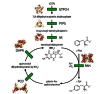
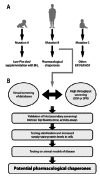
Phenylketonuria (PKU) is a loss-of-function inborn error of metabolism. As many other inherited diseases the main pathologic mechanism in PKU is an enhanced tendency of the mutant phenylalanine hydroxylase (PAH) to misfold and undergo ubiquitin-dependent degradation. Recent alternative approaches with therapeutic potential for PKU aim at correcting the PAH misfolding, and in this respect pharmacological chaperones are the focus of increasing interest. These compounds, which often resemble the natural ligands and show mild competitive inhibition, can rescue the misfolded proteins by stimulating their renaturation in vivo. For PKU, a few studies have proven the stabilization of PKU-mutants in vitro, in cells, and in mice by pharmacological chaperones, which have been found either by using the tetrahydrobiopterin (BH(4)) cofactor as query structure for shape-focused virtual screening or by high-throughput screening of small compound libraries. Both approaches have revealed a number of compounds, most of which bind at the iron-binding site, competitively with respect to BH(4). Furthermore, PAH shares a number of ligands, such as BH(4), amino acid substrates and inhibitors, with the other aromatic amino acid hydroxylases: the neuronal/neuroendocrine enzymes tyrosine hydroxylase (TH) and the tryptophan hydroxylases (TPHs). Recent results indicate that the PAH-targeted pharmacological chaperones should also be tested on TH and the TPHs, and eventually be derivatized to avoid unwanted interactions with these other enzymes. After derivatization and validation in animal models, the PAH-chaperoning compounds represent novel possibilities in the treatment of PKU.
Figures




Similar articles
-
Pharmacological Chaperones that Protect Tetrahydrobiopterin Dependent Aromatic Amino Acid Hydroxylases Through Different Mechanisms.Curr Drug Targets. 2016;17(13):1515-26. doi: 10.2174/1389450117666160307143512. Curr Drug Targets. 2016. PMID: 26953246 Review.
-
Novel pharmacological chaperones that correct phenylketonuria in mice.Hum Mol Genet. 2012 Apr 15;21(8):1877-87. doi: 10.1093/hmg/dds001. Epub 2012 Jan 13. Hum Mol Genet. 2012. PMID: 22246293
-
The G46S-hPAH mutant protein: a model to study the rescue of aggregation-prone PKU mutations by chaperones.Mol Genet Metab. 2011;104 Suppl:S40-4. doi: 10.1016/j.ymgme.2011.07.024. Epub 2011 Jul 31. Mol Genet Metab. 2011. PMID: 21871828 Review.
-
Pahenu1 is a mouse model for tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency and promotes analysis of the pharmacological chaperone mechanism in vivo.Hum Mol Genet. 2010 May 15;19(10):2039-49. doi: 10.1093/hmg/ddq085. Epub 2010 Feb 23. Hum Mol Genet. 2010. PMID: 20179079
-
New insights into tetrahydrobiopterin pharmacodynamics from Pah enu1/2, a mouse model for compound heterozygous tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency.Biochem Pharmacol. 2010 Nov 15;80(10):1563-71. doi: 10.1016/j.bcp.2010.07.042. Epub 2010 Aug 10. Biochem Pharmacol. 2010. PMID: 20705059
Cited by
-
Structural basis for ligand-dependent dimerization of phenylalanine hydroxylase regulatory domain.Sci Rep. 2016 Apr 6;6:23748. doi: 10.1038/srep23748. Sci Rep. 2016. PMID: 27049649 Free PMC article.
-
Structural mechanism for tyrosine hydroxylase inhibition by dopamine and reactivation by Ser40 phosphorylation.Nat Commun. 2022 Jan 10;13(1):74. doi: 10.1038/s41467-021-27657-y. Nat Commun. 2022. PMID: 35013193 Free PMC article.
-
Protein homeostasis defects of alanine-glyoxylate aminotransferase: new therapeutic strategies in primary hyperoxaluria type I.Biomed Res Int. 2013;2013:687658. doi: 10.1155/2013/687658. Epub 2013 Jul 16. Biomed Res Int. 2013. PMID: 23956997 Free PMC article. Review.
-
Second-Generation Pharmacological Chaperones: Beyond Inhibitors.Molecules. 2020 Jul 9;25(14):3145. doi: 10.3390/molecules25143145. Molecules. 2020. PMID: 32660097 Free PMC article. Review.
-
Engineering Organoids for in vitro Modeling of Phenylketonuria.Front Mol Neurosci. 2022 Jan 10;14:787242. doi: 10.3389/fnmol.2021.787242. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35082602 Free PMC article. Review.
References
-
- Fölling A. Über ausscheidung von phenylbrenztraubensäure in den harn als stoffwechselanomalie in verbindung mit imbezillitat. Hoppe-Seyl. 1934;227:169–176.
-
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet . 2010;376:1417–27. - PubMed
-
- van Spronsen FJ. Phenylketonuria: a 21st century perspective. Nat. Rev. Endocrinol. 2010;6:509–14. - PubMed
-
- de Groot M J, Hoeksma M, Blau N, Reijngoud D J, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria review of hypotheses. Mol. Genet. Metab. 2010;99(Suppl 1):S86–9. - PubMed
-
- Scriver CR. The PAH gene phenylketonuria and a paradigm shift. Hum. Mutat. 2007;28:831–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

