Mediator MED23 regulates basal transcription in vivo via an interaction with P-TEFb
- PMID: 23340209
- PMCID: PMC3644042
- DOI: 10.4161/trns.22874
Mediator MED23 regulates basal transcription in vivo via an interaction with P-TEFb
Abstract
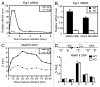
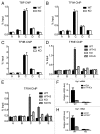
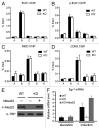
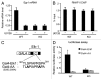
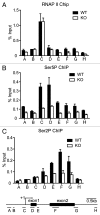
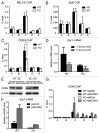
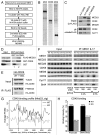
The Mediator is a multi-subunit complex that transduces regulatory information from transcription regulators to the RNA polymerase II apparatus. Growing evidence suggests that Mediator plays roles in multiple stages of eukaryotic transcription, including elongation. However, the detailed mechanism by which Mediator regulates elongation remains elusive. In this study, we demonstrate that Mediator MED23 subunit controls a basal level of transcription by recruiting elongation factor P-TEFb, via an interaction with its CDK9 subunit. The mRNA level of Egr1, a MED23-controlled model gene, is reduced 4-5 fold in Med23 (-/-) ES cells under an unstimulated condition, but Med23-deficiency does not alter the occupancies of RNAP II, GTFs, Mediator complex, or activator ELK1 at the Egr1 promoter. Instead, Med23 depletion results in a significant decrease in P-TEFb and RNAP II (Ser2P) binding at the coding region, but no changes for several other elongation regulators, such as DSIF and NELF. ChIP-seq revealed that Med23-deficiency partially reduced the P-TEFb occupancy at a set of MED23-regulated gene promoters. Further, we demonstrate that MED23 interacts with CDK9 in vivo and in vitro. Collectively, these results provide the mechanistic insight into how Mediator promotes RNAP II into transcription elongation.
Keywords: Egr1; Mediator MED23; P-TEFb; basal transcription; elongation.
Figures
Similar articles
-
The emerging picture of CDK9/P-TEFb: more than 20 years of advances since PITALRE.Mol Biosyst. 2017 Jan 31;13(2):246-276. doi: 10.1039/c6mb00387g. Mol Biosyst. 2017. PMID: 27833949 Review.
-
G-actin participates in RNA polymerase II-dependent transcription elongation by recruiting positive transcription elongation factor b (P-TEFb).J Biol Chem. 2011 Apr 29;286(17):15171-81. doi: 10.1074/jbc.M110.184374. Epub 2011 Mar 4. J Biol Chem. 2011. PMID: 21378166 Free PMC article.
-
P-TEFb, the super elongation complex and mediator regulate a subset of non-paused genes during early Drosophila embryo development.PLoS Genet. 2015 Feb 13;11(2):e1004971. doi: 10.1371/journal.pgen.1004971. eCollection 2015 Feb. PLoS Genet. 2015. PMID: 25679530 Free PMC article.
-
Bromodomain protein Brd4 regulates human immunodeficiency virus transcription through phosphorylation of CDK9 at threonine 29.J Virol. 2009 Jan;83(2):1036-44. doi: 10.1128/JVI.01316-08. Epub 2008 Oct 29. J Virol. 2009. PMID: 18971272 Free PMC article.
-
P-TEFb goes viral.Bioessays. 2016 Jul;38 Suppl 1:S75-85. doi: 10.1002/bies.201670912. Bioessays. 2016. PMID: 27417125 Review.
Cited by
-
Characterization of molecular and cellular functions of the cyclin-dependent kinase CDK9 using a novel specific inhibitor.Br J Pharmacol. 2014 Jan;171(1):55-68. doi: 10.1111/bph.12408. Br J Pharmacol. 2014. PMID: 24102143 Free PMC article.
-
Transcriptional complexity and roles of Fra-1/AP-1 at the uPA/Plau locus in aggressive breast cancer.Nucleic Acids Res. 2014;42(17):11011-24. doi: 10.1093/nar/gku814. Epub 2014 Sep 8. Nucleic Acids Res. 2014. PMID: 25200076 Free PMC article.
-
Liver Med23 ablation improves glucose and lipid metabolism through modulating FOXO1 activity.Cell Res. 2014 Oct;24(10):1250-65. doi: 10.1038/cr.2014.120. Epub 2014 Sep 16. Cell Res. 2014. PMID: 25223702 Free PMC article.
-
The Mediator subunit MED23 couples H2B mono-ubiquitination to transcriptional control and cell fate determination.EMBO J. 2015 Dec 2;34(23):2885-902. doi: 10.15252/embj.201591279. Epub 2015 Sep 1. EMBO J. 2015. PMID: 26330467 Free PMC article.
-
T-bet Activates Th1 Genes through Mediator and the Super Elongation Complex.Cell Rep. 2016 Jun 21;15(12):2756-70. doi: 10.1016/j.celrep.2016.05.054. Epub 2016 Jun 9. Cell Rep. 2016. PMID: 27292648 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
