Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice
- PMID: 23340250
- PMCID: PMC3589670
- DOI: 10.1210/me.2012-1286
Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice
Erratum in
-
Corrigendum. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice.Mol Endocrinol. 2015 Mar;29(3):484. doi: 10.1210/me.2015-1023. Mol Endocrinol. 2015. PMID: 25723485 Free PMC article. No abstract available.
Abstract
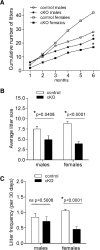
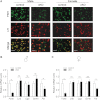
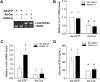
Impairments in pituitary FSH synthesis or action cause infertility. However, causes of FSH dysregulation are poorly described, in part because of our incomplete understanding of mechanisms controlling FSH synthesis. Previously, we discovered a critical role for forkhead protein L2 (FOXL2) in activin-stimulated FSH β-subunit (Fshb) transcription in immortalized cells in vitro. Here, we tested the hypothesis that FOXL2 is required for FSH synthesis in vivo. Using a Cre/lox approach, we selectively ablated Foxl2 in murine anterior pituitary gonadotrope cells. Conditional knockout (cKO) mice developed overtly normally but were subfertile in adulthood. Testis size and spermatogenesis were significantly impaired in cKO males. cKO females exhibited reduced ovarian weight and ovulated fewer oocytes in natural estrous cycles compared with controls. In contrast, ovaries of juvenile cKO females showed normal responses to exogenous gonadotropin stimulation. Both male and female cKO mice were FSH deficient, secondary to diminished pituitary Fshb mRNA production. Basal and activin-stimulated Fshb expression was similarly impaired in Foxl2 depleted primary pituitary cultures. Collectively, these data definitively establish FOXL2 as the first identified gonadotrope-restricted transcription factor required for selective FSH synthesis in vivo.
Figures
Similar articles
-
Human Follicle-Stimulating Hormone ß Subunit Expression Depends on FOXL2 and SMAD4.Endocrinology. 2020 May 1;161(5):bqaa045. doi: 10.1210/endocr/bqaa045. Endocrinology. 2020. PMID: 32191302 Free PMC article.
-
Impaired FSHbeta expression in the pituitaries of Foxl2 mutant animals.Mol Endocrinol. 2011 Aug;25(8):1404-15. doi: 10.1210/me.2011-0093. Epub 2011 Jun 23. Mol Endocrinol. 2011. PMID: 21700720 Free PMC article.
-
Normal gonadotropin production and fertility in gonadotrope-specific Bmpr1a knockout mice.J Endocrinol. 2016 Jun;229(3):331-41. doi: 10.1530/JOE-16-0053. Epub 2016 Mar 30. J Endocrinol. 2016. PMID: 27029473 Free PMC article.
-
Minireview: Activin Signaling in Gonadotropes: What Does the FOX say… to the SMAD?Mol Endocrinol. 2015 Jul;29(7):963-77. doi: 10.1210/me.2015-1004. Epub 2015 May 5. Mol Endocrinol. 2015. PMID: 25942106 Free PMC article. Review.
-
Mechanisms of activin-stimulated FSH synthesis: the story of a pig and a FOX.Biol Reprod. 2013 Mar 28;88(3):78. doi: 10.1095/biolreprod.113.107797. Print 2013 Mar. Biol Reprod. 2013. PMID: 23426431 Review.
Cited by
-
IGSF1 Does Not Regulate Spermatogenesis or Modify FSH Synthesis in Response to Inhibins or Activins.J Endocr Soc. 2021 Feb 20;5(4):bvab023. doi: 10.1210/jendso/bvab023. eCollection 2021 Apr 1. J Endocr Soc. 2021. PMID: 33796801 Free PMC article.
-
β-catenin stabilization in gonadotropes impairs FSH synthesis in male mice in vivo.Endocrinology. 2015 Jan;156(1):323-33. doi: 10.1210/en.2014-1296. Endocrinology. 2015. PMID: 25343272 Free PMC article.
-
Molecular regulation of follicle-stimulating hormone synthesis, secretion and action.J Mol Endocrinol. 2018 Apr;60(3):R131-R155. doi: 10.1530/JME-17-0308. Epub 2018 Feb 7. J Mol Endocrinol. 2018. PMID: 29437880 Free PMC article. Review.
-
Conditional Deletion of FOXL2 and SMAD4 in Gonadotropes of Adult Mice Causes Isolated FSH Deficiency.Endocrinology. 2018 Jul 1;159(7):2641-2655. doi: 10.1210/en.2018-00100. Endocrinology. 2018. PMID: 29800110 Free PMC article.
-
SMAD3 Regulates Follicle-stimulating Hormone Synthesis by Pituitary Gonadotrope Cells in Vivo.J Biol Chem. 2017 Feb 10;292(6):2301-2314. doi: 10.1074/jbc.M116.759167. Epub 2016 Dec 19. J Biol Chem. 2017. PMID: 27994055 Free PMC article.
References
-
- Aittomäki K, Dieguez Lucena J, Pakarinen P, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968 - PubMed
-
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583 - PubMed
-
- Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204 - PubMed
-
- Matthews CH, Borgato S, Beck-Peccoz P, et al. Primary amenorrhoea and infertility due to a mutation in the β-subunit of follicle-stimulating hormone. Nat Genet. 1993;5:83–86 - PubMed
-
- Layman LC, Lee EJ, Peak DB, et al. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone β-subunit gene. N Engl J Med. 1997;337:607–611 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

