IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells
- PMID: 23340251
- PMCID: PMC3589673
- DOI: 10.1210/me.2012-1307
IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells
Abstract
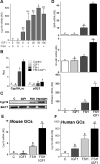
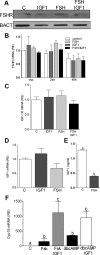
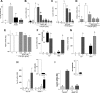
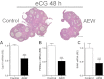
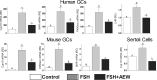
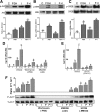
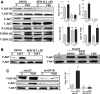
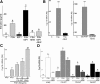
FSH and IGF-I synergistically stimulate gonadal steroid production; conversely, silencing the FSH or the IGF-I genes leads to infertility and hypogonadism. To determine the molecular link between these hormones, we examined the signaling cross talk downstream of their receptors. In human and rodent granulosa cells (GCs), IGF-I potentiated the stimulatory effects of FSH and cAMP on the expression of steroidogenic genes. In contrast, inhibition of IGF-I receptor (IGF-IR) activity or expression using pharmacological, genetic, or biochemical approaches prevented the FSH- and cAMP-induced expression of steroidogenic genes and estradiol production. In vivo experiments demonstrated that IGF-IR inactivation reduces the stimulation of steroidogenic genes and follicle growth by gonadotropins. FSH or IGF-I alone stimulated protein kinase B (PKB), which is also known as AKT and in combination synergistically increased AKT phosphorylation. Remarkably, blocking IGF-IR expression or activity decreased AKT basal activity and abolished AKT activation by FSH. In GCs lacking IGF-IR activity, FSH stimulation of Cyp19 expression was rescued by overexpression of constitutively active AKT. Our findings demonstrate, for the first time, that in human, mouse, and rat GCs, the well-known stimulatory effect of FSH on Cyp19 and AKT depends on IGF-I and on the expression and activation of the IGF-IR.
Figures
References
-
- Baker J, Hardy MP, Zhou J, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918 - PubMed
-
- Daftary SS, Gore AC. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med. 2005;230:292–306 - PubMed
-
- Hikake T, Hayashi S, Iguchi T, Sato T. The role of IGF1 on the differentiation of prolactin secreting cells in the mouse anterior pituitary. J Endocrinol. 2009;203:231–240 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

