One size does not fit all: the oligomeric states of αB crystallin
- PMID: 23340341
- PMCID: PMC3865782
- DOI: 10.1016/j.febslet.2013.01.021
One size does not fit all: the oligomeric states of αB crystallin
Abstract
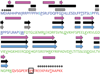
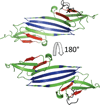
Small Heat Shock Proteins (sHSPs) are a diverse family of molecular chaperones that delay protein aggregation through interactions with non-native and aggregate-prone protein states. This function has been shown to be important to cellular viability and sHSP function/dysfunction is implicated in many diseases, including Alzheimer's and Alexander disease. Though their gene products are small, many sHSPs assemble into a distribution of large oligomeric states that undergo dynamic subunit exchange. These inherent properties present significant experimental challenges for characterizing sHSP oligomers. Of the human sHSPs, αB crystallin is a paradigm example of sHSP oligomeric properties. Advances in our understanding of sHSP structure, oligomeric distribution, and dynamics have prompted the proposal of several models for the oligomeric states of αB. The aim of this review is to highlight characteristics of αB crystallin (αB) that are key to understanding its structure and function. The current state of knowledge, existing models, and outstanding questions that remain to be addressed are presented.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;10:842–846. - PubMed
-
- Vicart P, Caron A, Guicheney P, Li Z, Prévost M, Faure A, Chateau D, Chapon F, Tomé F, Dupret J, Paulin D, Fardeau M. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. - PubMed
-
- Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, Mavros C, Coccia JA, Faget KY, Fitch KA, Masters CL, Tanzi RE, Jr, LTC, Bush AI. Cytosolic β-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet. 2003;361:1258–1265. - PubMed
-
- Kato K, Inaguma Y, Ito H, Iida K, Iwamoto I, Kamei K, Ochi N, Ohta H, Kishikawa M. Ser-59 is the major phosphorylation site in aBcrystallin accumulated in the brains of patients with Alexander’s disease. J Neurochem. 2001;76:730–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

