Behavioral, neurochemical and morphological changes induced by the overexpression of munc18-1a in brain of mice: relevance to schizophrenia
- PMID: 23340504
- PMCID: PMC3566728
- DOI: 10.1038/tp.2012.149
Behavioral, neurochemical and morphological changes induced by the overexpression of munc18-1a in brain of mice: relevance to schizophrenia
Abstract
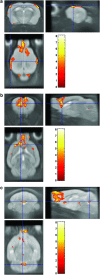
Overexpression of the mammalian homolog of the unc-18 gene (munc18-1) has been described in the brain of subjects with schizophrenia. Munc18-1 protein is involved in membrane fusion processes, exocytosis and neurotransmitter release. A transgenic mouse strain that overexpresses the protein isoform munc18-1a in the brain was characterized. This animal displays several schizophrenia-related behaviors, supersensitivity to hallucinogenic drugs and deficits in prepulse inhibition that reverse after antipsychotic treatment. Relevant brain areas (that is, cortex and striatum) exhibit reduced expression of dopamine D(1) receptors and dopamine transporters together with enhanced amphetamine-induced in vivo dopamine release. Magnetic resonance imaging demonstrates decreased gray matter volume in the transgenic animal. In conclusion, the mouse overexpressing brain munc18-1a represents a new valid animal model that resembles functional and structural abnormalities in patients with schizophrenia. The animal could provide valuable insights into phenotypic aspects of this psychiatric disorder.
Figures
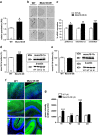
 , Ć). The specificity labeling was demonstrated by the displacement with unlabeled oligonucleotide (Á,
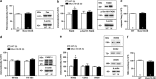
, Ć). The specificity labeling was demonstrated by the displacement with unlabeled oligonucleotide (Á,  , Ć). Note the increase of mRNA signal in cerebellum and piriform cortex. Pir, piriform cortex; CA1, CA1 field of hippocampus; CA3, CA3 field of hippocampus; DG, dentate gyrus; CB Cx, Cerebellum cortex. Bar: 2 mm. (c) Expression of mRNA encoding for munc18-1a in Munc18-OE and WT control mice evaluated by in situ hybridization. Significant increases of munc18-1a mRNA levels were obtained in different brain regions of Munc18-OE (unpaired t-test *P<0.05, **P<0.01 and ***P<0.005 versus WT control group). (d) Increased immunodensity of munc18-1a protein in the brain cortex of Munc18-OE mice (unpaired t-test **P<0.005 versus WT controls). Representative immunoblots of munc18-1a and β-actin in WT controls and Munc18-OE mice. (e) As a negative control, no differences were found in munc18-1b protein expression between Munc18-OE and WT controls. (f) Representative images of munc18-1a distribution in WT (left panels) and Munc18-OE (right panels) at 10 × , green (munc18-1a) and blue (nuclei) in cortex (cx), hippocampus (hp) and cerebellum (cb). (g) Increased immunofluorescence in different brain areas of Munc18-OE mice (unpaired t-test **P<0.005 and ***P<0.0001 versus WT controls); cx, cortex; hp, hippocampus; cb, cerebellum; str, striatum. Data are mean±s.e.m.; the number of animals is indicated in parentheses or inside plot bars.
, Ć). Note the increase of mRNA signal in cerebellum and piriform cortex. Pir, piriform cortex; CA1, CA1 field of hippocampus; CA3, CA3 field of hippocampus; DG, dentate gyrus; CB Cx, Cerebellum cortex. Bar: 2 mm. (c) Expression of mRNA encoding for munc18-1a in Munc18-OE and WT control mice evaluated by in situ hybridization. Significant increases of munc18-1a mRNA levels were obtained in different brain regions of Munc18-OE (unpaired t-test *P<0.05, **P<0.01 and ***P<0.005 versus WT control group). (d) Increased immunodensity of munc18-1a protein in the brain cortex of Munc18-OE mice (unpaired t-test **P<0.005 versus WT controls). Representative immunoblots of munc18-1a and β-actin in WT controls and Munc18-OE mice. (e) As a negative control, no differences were found in munc18-1b protein expression between Munc18-OE and WT controls. (f) Representative images of munc18-1a distribution in WT (left panels) and Munc18-OE (right panels) at 10 × , green (munc18-1a) and blue (nuclei) in cortex (cx), hippocampus (hp) and cerebellum (cb). (g) Increased immunofluorescence in different brain areas of Munc18-OE mice (unpaired t-test **P<0.005 and ***P<0.0001 versus WT controls); cx, cortex; hp, hippocampus; cb, cerebellum; str, striatum. Data are mean±s.e.m.; the number of animals is indicated in parentheses or inside plot bars.
Similar articles
-
Regulation of munc18-1 and syntaxin-1A interactive partners in schizophrenia prefrontal cortex: down-regulation of munc18-1a isoform and 75 kDa SNARE complex after antipsychotic treatment.Int J Neuropsychopharmacol. 2012 Jun;15(5):573-88. doi: 10.1017/S1461145711000861. Epub 2011 Jun 14. Int J Neuropsychopharmacol. 2012. PMID: 21669024
-
Cytokine pathway disruption in a mouse model of schizophrenia induced by Munc18-1a overexpression in the brain.J Neuroinflammation. 2014 Jul 29;11:128. doi: 10.1186/1742-2094-11-128. J Neuroinflammation. 2014. PMID: 25069615 Free PMC article.
-
Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia.Genes Brain Behav. 2010 Oct;9(7):777-89. doi: 10.1111/j.1601-183X.2010.00615.x. Epub 2010 Aug 12. Genes Brain Behav. 2010. PMID: 20618446
-
Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects.Schizophr Bull. 2010 Mar;36(2):246-70. doi: 10.1093/schbul/sbp132. Epub 2009 Nov 9. Schizophr Bull. 2010. PMID: 19900963 Free PMC article. Review.
-
The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged?Annu Rev Cell Dev Biol. 2012;28:279-308. doi: 10.1146/annurev-cellbio-101011-155818. Annu Rev Cell Dev Biol. 2012. PMID: 23057743 Review.
Cited by
-
The role of snare proteins in cortical development.Dev Neurobiol. 2022 Sep;82(6):457-475. doi: 10.1002/dneu.22892. Epub 2022 Jul 5. Dev Neurobiol. 2022. PMID: 35724379 Free PMC article. Review.
-
Proteome Analysis of Potential Synaptic Vesicle Cycle Biomarkers in the Cerebrospinal Fluid of Patients with Sporadic Creutzfeldt-Jakob Disease.Mol Neurobiol. 2017 Sep;54(7):5177-5191. doi: 10.1007/s12035-016-0029-6. Epub 2016 Aug 25. Mol Neurobiol. 2017. PMID: 27562179
-
The Absence of Caspase-8 in the Dopaminergic System Leads to Mild Autism-like Behavior.Front Cell Dev Biol. 2022 Apr 5;10:839715. doi: 10.3389/fcell.2022.839715. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493109 Free PMC article.
-
Reduced SNAP25 Protein Fragmentation Contributes to SNARE Complex Dysregulation in Schizophrenia Postmortem Brain.Neuroscience. 2019 Nov 10;420:112-128. doi: 10.1016/j.neuroscience.2018.12.015. Epub 2018 Dec 21. Neuroscience. 2019. PMID: 30579835 Free PMC article.
-
Proteogenomics Reveals Orthologous Alternatively Spliced Proteoforms in the Same Human and Mouse Brain Regions with Differential Abundance in an Alzheimer's Disease Mouse Model.Cells. 2021 Jun 23;10(7):1583. doi: 10.3390/cells10071583. Cells. 2021. PMID: 34201730 Free PMC article.
References
-
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. - PubMed
-
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. - PubMed
-
- Arango C, Rapado-Castro M, Reig S, Castro-Fornieles J, Gonzalez-Pinto A, Otero S, et al. Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry. 2012;69:16–26. - PubMed
-
- Johnson RD, Oliver PL, Davies KE. SNARE proteins and schizophrenia: linking synaptic and neurodevelopmental hypotheses. Acta Biochim Pol. 2008;55:619–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

