Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK)
- PMID: 23340516
- PMCID: PMC3778339
- DOI: 10.1038/ejhg.2012.301
Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK)
Abstract
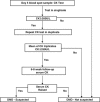
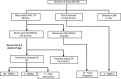
Duchenne muscular dystrophy (DMD), a progressive X-linked neuromuscular disorder, has an estimated worldwide incidence of 1:3500 male births. Currently, there are no curative treatments and the mean age of diagnosis is 5 years. In addition, subsequent pregnancies frequently occur before a diagnosis is made in an index case. An 'opt in' screening programme was introduced in Wales in 1990 with the aim to: reduce the diagnostic delay, permit reproductive choice and allow planning of the care of the affected boy. Newborn bloodspots were collected routinely as part of the Wales newborn screening programme. Specific consent was obtained for this test separately from the other tests. During the 21-year period, 369,780 bloodspot cards were received from male infants, of these 343,170 (92.8%) were screened using a bloodspot creatine kinase (CK) assay following parental consent. A total of 145 cases had a raised CK activity (≥250 U/l) and at follow-up, at 6-8 weeks of age, 79 cases had a normal serum CK (false-positive rate 0.023%) and 66 cases had an elevated serum CK. DMD was confirmed in 56 cases by genotyping/muscle biopsy studies, Becker muscular dystrophy in 5 cases and other rarer forms of muscular dystrophy in 5 cases. This long-term study has so far identified 13 false-negative cases. The incidence of DMD in Wales of 1:5136 during this period is lower than that of 1:4046 before commencement of screening in Wales. Screening has reduced the diagnostic delay enabling reproductive choice for parents of affected boys and earlier administration of current therapies.
Figures
References
-
- Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromusc Disord. 1991;1:19–29. - PubMed
-
- Mohamed K, Appleton R, Nicolaides P. Delayed diagnosis of Duchenne muscular dystrophy. Eur J of Paed Neurol. 2000;4:219–223. - PubMed
-
- Bushby K, Hill A, Steele J. Failure of early diagnosis in symptomatic duchenne muscular dystrophy. Lancet. 1999;353:557. - PubMed
-
- Parsons EP, Clarke AJ, Bradley DM. Developmental progress in Duchenne muscular dystrophy: lessons for earlier detection. Eur J Paed Neurol. 2004;8:145–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials