Improved vancomycin dosing in children using area under the curve exposure
- PMID: 23340565
- PMCID: PMC3632448
- DOI: 10.1097/INF.0b013e318286378e
Improved vancomycin dosing in children using area under the curve exposure
Abstract
Background: : Our objectives were to (1) determine the pharmacokinetic indices of vancomycin in pediatric patients; and (2) compare attainment of 2 target exposures: area under curve (AUC) / minimum inhibitory concentration (MIC) ≥400 and trough concentration ≥15 mcg/mL.
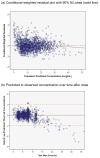
Methods: : The population-based pharmacokinetic modeling was performed using NONMEM 7.2 for children ≥3 months old who received vancomycin for ≥48 hours from 2003 to 2011. A 1-compartment model with first-order kinetics was used to estimate clearance, volume of distribution and AUC. Empiric Bayesian post hoc individual parameters and Monte Carlo simulations (N = 11,000) were performed.
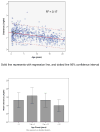
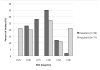
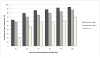
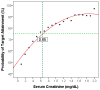
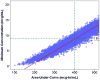
Results: : Analysis included 702 patients with 1660 vancomycin serum concentrations. Median age was 6.6 (interquartile range 2.2-13.4) years, weight 22.7 (12.6-46) kg and baseline serum creatinine 0.40 (0.30-0.60) mg/dL. Final model pharmacokinetic indices were clearance (L/h) = 0.248 * Wt * (0.48/serum creatinine) * (ln(age)/7.8) and volume of distribution (L) = 0.636 * Wt. Using these parameters and the observed MIC distribution, Monte Carlo simulation indicated that the initial median dose of 44 (39-52) mg/kg/day was inadequate in most subjects. Regimens of 60 mg/kg/day for subjects ≥12 years old and 70 mg/kg/day for those <12 years old achieved target AUC/MIC in ~75% and trough concentrations ≥15 in ~45% of virtual subjects. An AUC/MIC ~400 corresponded to trough concentration ~8 to 9 mcg/mL.
Conclusions: : Targeted exposure using vancomycin AUC/MIC, compared with trough concentrations, is a more realistic target in children. Depending on age, serum creatinine and MIC distribution, vancomycin in a dosage of 60 to 70 mg/kg/day was necessary to achieve AUC/MIC ≥ 400 in 75% of patients.
Conflict of interest statement
Figures



References
-
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. - PubMed
-
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. - PubMed
-
- Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52(8):975–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

