SU9518 inhibits proliferative vitreoretinopathy in fibroblast and genetically modified Müller cell-induced rabbit models
- PMID: 23341018
- PMCID: PMC3597189
- DOI: 10.1167/iovs.12-10320
SU9518 inhibits proliferative vitreoretinopathy in fibroblast and genetically modified Müller cell-induced rabbit models
Abstract
Purpose: Proliferative vitreoretinopathy (PVR) is a complication of retinal detachment that can lead to surgical failure and vision loss. Previous studies suggest that a variety of retinal cells, including RPE and Müller glia, may be responsible. Platelet-derived growth factor receptor alpha (PDGFRα) has been strongly implicated in the pathogenesis, and found to be intrinsic to the development of PVR in rabbit models. We examine whether SU9518, a tyrosine kinase inhibitor with PDGFRα specificity, can inhibit the development of PVR in fibroblast and Müller cell rabbit models of PVR.
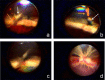
Methods: SU9518 was injected in rabbit eyes along with fibroblasts, Müller cells (MIO-M1), or Müller cells transfected to increase their expression of PDGFRα (MIO-M1α). Indirect ophthalmoscopy and histopathology were used to assess efficacy and toxicity.
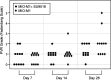
Results: SU9518 was an effective inhibitor of PVR in both fibroblast and Müller cell models of PVR. No toxic effects were identified by indirect ophthalmoscopy or histopathology.
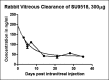
Conclusions: SU9518 is an effective and safe inhibitor of PVR in rabbit models, and could potentially be used in humans for the treatment of this and other proliferative diseases of the retina involving fibrosis and gliosis. Further animal studies need to be performed to examine retinal toxicity and sustained delivery mechanisms.
Conflict of interest statement
Disclosure:
Figures





Similar articles
-
Retinal Pigment Epithelium and Müller Progenitor Cell Interaction Increase Müller Progenitor Cell Expression of PDGFRα and Ability to Induce Proliferative Vitreoretinopathy in a Rabbit Model.Stem Cells Int. 2012;2012:106486. doi: 10.1155/2012/106486. Epub 2012 Aug 23. Stem Cells Int. 2012. PMID: 22966235 Free PMC article.
-
Evaluation of the Effect of Everolimus on Retinal Pigment Epithelial Cells and Experimental Proliferative Vitreoretinopathy.Curr Eye Res. 2018 Mar;43(3):333-339. doi: 10.1080/02713683.2017.1396618. Epub 2017 Nov 28. Curr Eye Res. 2018. PMID: 29182404
-
Ribozyme to proliferating cell nuclear antigen to treat proliferative vitreoretinopathy.Invest Ophthalmol Vis Sci. 2002 Oct;43(10):3338-48. Invest Ophthalmol Vis Sci. 2002. PMID: 12356843
-
[Periostin in the Pathogenesis of Proliferative Vitreoretinopathy].Nippon Ganka Gakkai Zasshi. 2015 Nov;119(11):772-80. Nippon Ganka Gakkai Zasshi. 2015. PMID: 26685481 Review. Japanese.
-
Is neutralizing vitreal growth factors a viable strategy to prevent proliferative vitreoretinopathy?Prog Retin Eye Res. 2014 May;40:16-34. doi: 10.1016/j.preteyeres.2013.12.006. Epub 2014 Jan 9. Prog Retin Eye Res. 2014. PMID: 24412519 Review.
Cited by
-
Epithelial-to-Mesenchymal Transition of RPE Cells In Vitro Confers Increased β1,6-N-Glycosylation and Increased Susceptibility to Galectin-3 Binding.PLoS One. 2016 Jan 13;11(1):e0146887. doi: 10.1371/journal.pone.0146887. eCollection 2016. PLoS One. 2016. PMID: 26760037 Free PMC article.
-
Arsenic trioxide inhibits proliferation of retinal pigment epithelium by downregulating expression of extracellular matrix and p27.Int J Clin Exp Pathol. 2020 Feb 1;13(2):172-178. eCollection 2020. Int J Clin Exp Pathol. 2020. PMID: 32211097 Free PMC article.
-
[Pharmacological approach to treatment of proliferative vitreoretinopathy].Ophthalmologe. 2013 Oct;110(10):948-59. doi: 10.1007/s00347-013-2832-z. Ophthalmologe. 2013. PMID: 24046169 Review. German.
-
Postoperative Macular Proliferative Vitreoretinopathy: A Case Series and Literature Review.Case Rep Ophthalmol. 2021 May 27;12(2):464-472. doi: 10.1159/000512285. eCollection 2021 May-Aug. Case Rep Ophthalmol. 2021. PMID: 34177543 Free PMC article.
-
Transition to Chronic Fibrosis in an Animal Model of Retinal Detachment With Features of Proliferative Vitreoretinopathy.Invest Ophthalmol Vis Sci. 2023 Dec 1;64(15):39. doi: 10.1167/iovs.64.15.39. Invest Ophthalmol Vis Sci. 2023. PMID: 38153753 Free PMC article.
References
-
- Zilis JD, Machemer R. Light damage in detached retina. Am J Ophthalmol. 1991; 111: 47–50 - PubMed
-
- Fisher SK, Erickson PA, Lewis GP, Anderson DH. Intraretinal proliferation induced by retinal detachment. Invest Ophthalmol Vis Sci. 1991; 32: 1739–1748 - PubMed
-
- Lewis GP, Charteris DG, Sethi CS, Leitner WP, Linberg KA, Fisher SK. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002; 43: 2412–2420 - PubMed
-
- Weller M, Wiedemann P, Heimann K. Proliferative vitreoretinopathy—is it anything more than wound healing at the wrong place? Int Ophthalmol. 1990; 14: 105–117 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

