Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils
- PMID: 23341359
- PMCID: PMC3585601
- DOI: 10.1104/pp.112.208934
Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils
Abstract
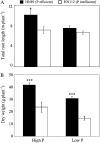
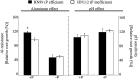
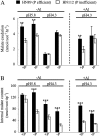
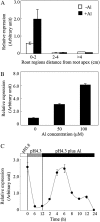
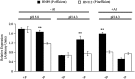
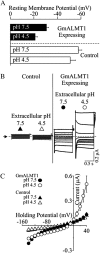
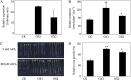
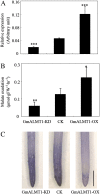
Low pH, aluminum (Al) toxicity, and low phosphorus (P) often coexist and are heterogeneously distributed in acid soils. To date, the underlying mechanisms of crop adaptation to these multiple factors on acid soils remain poorly understood. In this study, we found that P addition to acid soils could stimulate Al tolerance, especially for the P-efficient genotype HN89. Subsequent hydroponic studies demonstrated that solution pH, Al, and P levels coordinately altered soybean (Glycine max) root growth and malate exudation. Interestingly, HN89 released more malate under conditions mimicking acid soils (low pH, +P, and +Al), suggesting that root malate exudation might be critical for soybean adaptation to both Al toxicity and P deficiency on acid soils. GmALMT1, a soybean malate transporter gene, was cloned from the Al-treated root tips of HN89. Like root malate exudation, GmALMT1 expression was also pH dependent, being suppressed by low pH but enhanced by Al plus P addition in roots of HN89. Quantitative real-time PCR, transient expression of a GmALMT1-yellow fluorescent protein chimera in Arabidopsis protoplasts, and electrophysiological analysis of Xenopus laevis oocytes expressing GmALMT1 demonstrated that GmALMT1 encodes a root cell plasma membrane transporter that mediates malate efflux in an extracellular pH-dependent and Al-independent manner. Overexpression of GmALMT1 in transgenic Arabidopsis, as well as overexpression and knockdown of GmALMT1 in transgenic soybean hairy roots, indicated that GmALMT1-mediated root malate efflux does underlie soybean Al tolerance. Taken together, our results suggest that malate exudation is an important component of soybean adaptation to acid soils and is coordinately regulated by three factors, pH, Al, and P, through the regulation of GmALMT1 expression and GmALMT1 function.
Figures

Similar articles
-
Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system.Plant Physiol. 2006 Jun;141(2):674-84. doi: 10.1104/pp.105.076497. Epub 2006 Apr 28. Plant Physiol. 2006. PMID: 16648222 Free PMC article.
-
Maize ZmALMT2 is a root anion transporter that mediates constitutive root malate efflux.Plant Cell Environ. 2012 Jul;35(7):1185-200. doi: 10.1111/j.1365-3040.2011.02479.x. Epub 2012 Jan 17. Plant Cell Environ. 2012. PMID: 22211473
-
A domain-based approach for analyzing the function of aluminum-activated malate transporters from wheat (Triticum aestivum) and Arabidopsis thaliana in Xenopus oocytes.Plant Cell Physiol. 2014 Dec;55(12):2126-38. doi: 10.1093/pcp/pcu143. Epub 2014 Oct 13. Plant Cell Physiol. 2014. PMID: 25311199
-
Aluminum phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies.Ecotoxicol Environ Saf. 2024 Jan 1;269:115791. doi: 10.1016/j.ecoenv.2023.115791. Epub 2023 Dec 8. Ecotoxicol Environ Saf. 2024. PMID: 38070417 Review.
-
Molecular and physiological strategies to increase aluminum resistance in plants.Mol Biol Rep. 2012 Mar;39(3):2069-79. doi: 10.1007/s11033-011-0954-4. Epub 2011 Jun 10. Mol Biol Rep. 2012. PMID: 21660471 Review.
Cited by
-
The varied functions of aluminium-activated malate transporters-much more than aluminium resistance.Biochem Soc Trans. 2016 Jun 15;44(3):856-62. doi: 10.1042/BST20160027. Biochem Soc Trans. 2016. PMID: 27284052 Free PMC article. Review.
-
The Formation of Hollow Trait in Cucumber (Cucumis sativus L.) Fruit Is Controlled by CsALMT2.Int J Mol Sci. 2022 May 31;23(11):6173. doi: 10.3390/ijms23116173. Int J Mol Sci. 2022. PMID: 35682858 Free PMC article.
-
GmWRKY81 Encoding a WRKY Transcription Factor Enhances Aluminum Tolerance in Soybean.Int J Mol Sci. 2022 Jun 10;23(12):6518. doi: 10.3390/ijms23126518. Int J Mol Sci. 2022. PMID: 35742961 Free PMC article.
-
Comparative characterization of GmSPX members reveals that GmSPX3 is involved in phosphate homeostasis in soybean.Ann Bot. 2014 Sep;114(3):477-88. doi: 10.1093/aob/mcu147. Epub 2014 Jul 29. Ann Bot. 2014. PMID: 25074550 Free PMC article.
-
Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean.BMC Genomics. 2016 Mar 11;17:223. doi: 10.1186/s12864-016-2559-8. BMC Genomics. 2016. PMID: 26968518 Free PMC article.
References
-
- Archambault DJ, Zhang GC, Taylor GJ. (1997) Spatial variation in the kinetics of aluminium (Al) uptake in roots of wheat (Triticum aestivum L.) exhibiting differential resistance to Al: evidence for metabolism dependent exclusion of Al. J Plant Physiol 151: 668–674
-
- Basu U, Good AG, Aung T, Slaski J, Basu A, Briggs KG, Taylor GJ. (1999) A 23-kDa root exudates polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physiol Plant 106: 53–61
-
- Bertin C, Yang X, Weston L. (2003) The role of root exudates and allelo chemicals in the rhizosphere. Plant Soil 256: 67–83
-
- Cranados G, Pandey S, Ceballos H. (1992) Response to selection for tolerance to acid soils in a tropical maize population. Crop Sci 33: 936–940
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

