Novel sexual-cycle-specific gene silencing in Aspergillus nidulans
- PMID: 23341415
- PMCID: PMC3606093
- DOI: 10.1534/genetics.112.147546
Novel sexual-cycle-specific gene silencing in Aspergillus nidulans
Abstract
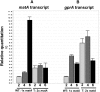
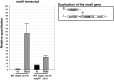
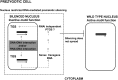
We report a novel sexual-cycle-specific gene-silencing system in the genetic model Aspergillus nidulans. Duplication of the mating type matA(HMG) gene in this haploid organism triggers Mat-induced silencing (MatIS) of both endogenous and transgenic matA genes, eliminates function of the encoded SRY structural ortholog, and results in formation of barren fruiting bodies. MatIS is spatiotemporally restricted to the prezygotic stage of the sexual cycle and does not interfere with vegetative growth, asexual reproduction, differentiation of early sexual tissues, or fruiting body development. MatIS is reversible upon deletion of the matA transgene. In contrast to other sex-specific silencing phenomena, MatIS silencing has nearly 100% efficiency and appears to be independent of homologous duplicated DNA segments. Remarkably, transgene-derived matA RNA might be sufficient to induce MatIS. A unique feature of MatIS is that RNA-mediated silencing is RNA interference/Argonaute-independent and is restricted to the nucleus having the duplicated gene. The silencing phenomenon is recessive and does not spread between nuclei within the common cytoplasm of a multinucleate heterokaryon. Gene silencing induced by matA gene duplication emerges as a specific feature associated with matA(HMG) regulation during sexual development.
Figures




Similar articles
-
Complex mechanisms regulate developmental expression of the matA (HMG) mating type gene in homothallic Aspergillus nidulans.Genetics. 2011 Nov;189(3):795-808. doi: 10.1534/genetics.111.131458. Epub 2011 Aug 25. Genetics. 2011. PMID: 21868608 Free PMC article.
-
Structural and functional conservation of fungal MatA and human SRY sex-determining proteins.Nat Commun. 2014 Nov 17;5:5434. doi: 10.1038/ncomms6434. Nat Commun. 2014. PMID: 25399555 Free PMC article.
-
Mating type protein Mat1-2 from asexual Aspergillus fumigatus drives sexual reproduction in fertile Aspergillus nidulans.Eukaryot Cell. 2008 Jun;7(6):1029-40. doi: 10.1128/EC.00380-07. Epub 2008 Feb 1. Eukaryot Cell. 2008. PMID: 18245277 Free PMC article.
-
Roles for receptors, pheromones, G proteins, and mating type genes during sexual reproduction in Neurospora crassa.Genetics. 2012 Apr;190(4):1389-404. doi: 10.1534/genetics.111.136358. Epub 2012 Jan 31. Genetics. 2012. PMID: 22298702 Free PMC article.
-
Genetic regulation of development in Aspergillus nidulans.Trends Genet. 1988 Jun;4(6):162-9. doi: 10.1016/0168-9525(88)90022-4. Trends Genet. 1988. PMID: 3076298 Review. No abstract available.
Cited by
-
Permissiveness and competition within and between Neurospora crassa syncytia.Genetics. 2023 Aug 9;224(4):iyad112. doi: 10.1093/genetics/iyad112. Genetics. 2023. PMID: 37313736 Free PMC article.
-
Are any fungal genes nucleus-limited?J Biosci. 2014 Jun;39(3):341-6. doi: 10.1007/s12038-014-9419-y. J Biosci. 2014. PMID: 24845498 No abstract available.
-
Neurospora Heterokaryons with Complementary Duplications and Deficiencies in Their Constituent Nuclei Provide an Approach to Identify Nucleus-Limited Genes.G3 (Bethesda). 2015 Apr 20;5(6):1263-72. doi: 10.1534/g3.115.017616. G3 (Bethesda). 2015. PMID: 25897010 Free PMC article.
-
RNAi function, diversity, and loss in the fungal kingdom.Chromosome Res. 2013 Dec;21(6-7):561-72. doi: 10.1007/s10577-013-9388-2. Chromosome Res. 2013. PMID: 24173579 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

