The polysialyltransferases interact with sequences in two domains of the neural cell adhesion molecule to allow its polysialylation
- PMID: 23341449
- PMCID: PMC3591636
- DOI: 10.1074/jbc.M112.438374
The polysialyltransferases interact with sequences in two domains of the neural cell adhesion molecule to allow its polysialylation
Abstract
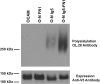
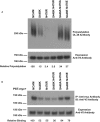
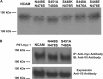
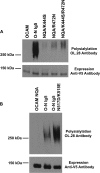
The neural cell adhesion molecule (NCAM) is the major substrate for the polysialyltransferases (polySTs), ST8SiaII/STX and ST8SiaIV/PST. The polysialylation of NCAM N-glycans decreases cell adhesion and alters signaling. Previous work demonstrated that the first fibronectin type III repeat (FN1) of NCAM is required for polyST recognition and the polysialylation of the N-glycans on the adjacent Ig5 domain. In this work, we highlight the importance of an FN1 acidic patch in polyST recognition and also reveal that the polySTs are required to interact with sequences in the Ig5 domain for polysialylation to occur. We find that features of the Ig5 domain of the olfactory cell adhesion molecule (OCAM) are responsible for its lack of polysialylation. Specifically, two basic OCAM Ig5 residues (Lys and Arg) found near asparagines equivalent to those carrying the polysialylated N-glycans in NCAM substantially decrease or eliminate polysialylation when used to replace the smaller and more neutral residues (Ser and Asn) in analogous positions in NCAM Ig5. This decrease in polysialylation does not reflect altered glycosylation but instead is correlated with a decrease in polyST-NCAM binding. In addition, inserting non-conserved OCAM sequences into NCAM Ig5, including an "extra" N-glycosylation site, decreases or completely blocks NCAM polysialylation. Taken together, these results indicate that the polySTs not only recognize an acidic patch in the FN1 domain of NCAM but also must contact sequences in the Ig5 domain for polysialylation of Ig5 N-glycans to occur.
Figures


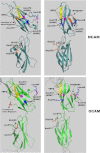
References
-
- Haltiwanger R. S., Lowe J. B. (2004) Role of glycosylation in development. Annu. Rev. Biochem. 73, 491–537 - PubMed
-
- Johnson C. P., Fujimoto I., Rutishauser U., Leckband D. E. (2005) Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J. Biol. Chem. 280, 137–145 - PubMed
-
- Hildebrandt H., Mühlenhoff M., Weinhold B., Gerardy-Schahn R. (2007) Dissecting polysialic acid and NCAM functions in brain development. J. Neurochem. 103, 56–64 - PubMed
-
- Rutishauser U. (2008) Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 9, 26–35 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

