Autophagy protects the retina from light-induced degeneration
- PMID: 23341467
- PMCID: PMC3597791
- DOI: 10.1074/jbc.M112.439935
Autophagy protects the retina from light-induced degeneration
Abstract
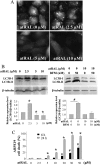
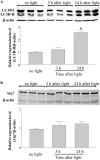
Autophagy is a conserved feature of lysosome-mediated intracellular degradation. Dysregulated autophagy is implicated as a contributor in neurodegenerative diseases; however, the role of autophagy in retinal degeneration remains largely unknown. Here, we report that the photo-activated visual chromophore, all-trans-retinal, modulated autophagosome formation in ARPE19 retinal cells. Increased formation of autophagosomes in these cells was observed when incubated with 2.5 μM all-trans-retinal, a condition that did not cause cell death after 24 h in culture. However, autophagosome formation was decreased at concentrations, which caused cell death. Increased expression of activating transcription factor 4 (Atf4), which indicates the activation of oxidative stress, was recorded in response to light illumination in retinas of Abca4(-/-)Rdh8(-/-) mice, which showed delayed clearance of all-trans-retinal after light exposure. Expression of autophagosome marker LC3B-II and mitochondria-specific autophagy, mitophagy, regulator Park2, were significantly increased in the retinas of Abca4(-/-)Rdh8(-/-) mice after light exposure, suggesting involvement of autophagy and mitophagy in the pathogenesis of light-induced retinal degeneration. Deletion of essential genes required for autophagy, including Beclin1 systemically or Atg7 in only rod photoreceptors resulted in increased susceptibility to light-induced retinal damage. Increased photoreceptor cell death was observed when retinas lacking the rod photoreceptor-specific Atg7 gene were coincubated with 20 μM all-trans-retinal. Park2(-/-) mice also displayed light-induced retinal degeneration. Ultra-structural analyses showed mitochondrial and endoplasmic reticulum impairment in retinas of these model animals after light exposure. Taken together, these observations provide novel evidence implicating an important role of autophagy and mitophagy in protecting the retina from all-trans-retinal- and light-induced degeneration.
Figures







Similar articles
-
Retinal cone and rod photoreceptor cells exhibit differential susceptibility to light-induced damage.J Neurochem. 2012 Apr;121(1):146-56. doi: 10.1111/j.1471-4159.2012.07647.x. Epub 2012 Feb 9. J Neurochem. 2012. PMID: 22220722 Free PMC article.
-
Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice.J Biol Chem. 2011 Apr 29;286(17):15543-55. doi: 10.1074/jbc.M111.228551. Epub 2011 Mar 7. J Biol Chem. 2011. PMID: 21383019 Free PMC article.
-
Limited roles of Rdh8, Rdh12, and Abca4 in all-trans-retinal clearance in mouse retina.Invest Ophthalmol Vis Sci. 2009 Nov;50(11):5435-43. doi: 10.1167/iovs.09-3944. Epub 2009 Jun 24. Invest Ophthalmol Vis Sci. 2009. PMID: 19553623 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Retinal photodamage mediated by all-trans-retinal.Photochem Photobiol. 2012 Nov-Dec;88(6):1309-19. doi: 10.1111/j.1751-1097.2012.01143.x. Epub 2012 Apr 24. Photochem Photobiol. 2012. PMID: 22428905 Free PMC article. Review.
Cited by
-
Foxg1-Cre Mediated Lrp2 Inactivation in the Developing Mouse Neural Retina, Ciliary and Retinal Pigment Epithelia Models Congenital High Myopia.PLoS One. 2015 Jun 24;10(6):e0129518. doi: 10.1371/journal.pone.0129518. eCollection 2015. PLoS One. 2015. PMID: 26107939 Free PMC article.
-
Intravenous route to choroidal neovascularization by macrophage-disguised nanocarriers for mTOR modulation.Acta Pharm Sin B. 2022 May;12(5):2506-2521. doi: 10.1016/j.apsb.2021.10.022. Epub 2021 Oct 28. Acta Pharm Sin B. 2022. PMID: 35646523 Free PMC article.
-
Increased susceptibility to fundus camera-delivered light-induced retinal degeneration in mice deficient in oxidative stress response proteins.Exp Eye Res. 2017 Jun;159:58-68. doi: 10.1016/j.exer.2017.03.009. Epub 2017 Mar 20. Exp Eye Res. 2017. PMID: 28336262 Free PMC article.
-
Myelinosome organelles in pathological retinas: ubiquitous presence and dual role in ocular proteostasis maintenance.Neural Regen Res. 2023 May;18(5):1009-1016. doi: 10.4103/1673-5374.355753. Neural Regen Res. 2023. PMID: 36254982 Free PMC article. Review.
-
Single Cell RNA Sequencing Analysis of Mouse Retina Identifies a Subpopulation of Muller Glia Involved in Retinal Recovery From Injury in the FCD-LIRD Model.Invest Ophthalmol Vis Sci. 2023 Aug 1;64(11):2. doi: 10.1167/iovs.64.11.2. Invest Ophthalmol Vis Sci. 2023. PMID: 37526616 Free PMC article.
References
-
- Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P. A., Small S., Spencer B., Rockenstein E., Levine B., Wyss-Coray T. (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Invest. 118, 2190–2199 - PMC - PubMed
-
- Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., Mizushima N. (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 - PubMed
-
- Mizushima N., Komatsu M. (2011) Autophagy. Renovation of cells and tissues. Cell 147, 728–741 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

