Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results
- PMID: 23341531
- PMCID: PMC4820756
- DOI: 10.1200/JCO.2012.44.7516
Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results
Abstract
Purpose: TNFerade biologic is a novel means of delivering tumor necrosis factor alpha to tumor cells by gene transfer. We herein report final results of the largest randomized phase III trial performed to date among patients with locally advanced pancreatic cancer (LAPC) and the first to test gene transfer against this malignancy.
Patients and methods: In all, 304 patients were randomly assigned 2:1 to standard of care plus TNFerade (SOC + TNFerade) versus standard of care alone (SOC). SOC consisted of 50.4 Gy in 28 fractions with concurrent fluorouracil (200 mg/m(2) per day continuous infusion). TNFerade was injected intratumorally before the first fraction of radiotherapy each week at a dose of 4 × 10(11) particle units by using either a percutaneous transabdominal or an endoscopic ultrasound approach. Four weeks after chemoradiotherapy, patients began gemcitabine (1,000 mg/m(2) intravenously) with or without erlotinib (100 to 150 mg per day orally) until progression or toxicity.
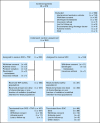
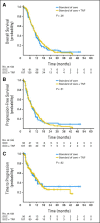
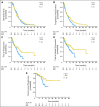
Results: The analysis included 187 patients randomly assigned to SOC + TNFerade and 90 to SOC by using a modified intention-to-treat approach. Median follow-up was 9.1 months (range, 0.1 to 50.5 months). Median survival was 10.0 months for patients in both the SOC + TNFerade and SOC arms (hazard ratio [HR], 0.90; 95% CI, 0.66 to 1.22; P = .26). Median progression-free survival (PFS) was 6.8 months for SOC + TNFerade versus 7.0 months for SOC (HR, 0.96; 95% CI, 0.69 to 1.32; P = .51). Among patients treated on the SOC + TNFerade arm, multivariate analysis showed that TNFerade injection by an endoscopic ultrasound-guided transgastric/transduodenal approach rather than a percutaneous transabdominal approach was a risk factor for inferior PFS (HR, 2.08; 95% CI, 1.06 to 4.06; P = .032). The patients in the SOC + TNFerade arm experienced more grade 1 to 2 fever and chills than those in the SOC arm (P < .001) but both arms had similar rates of grade 3 to 4 toxicities (all P > .05).
Conclusion: SOC + TNFerade is safe but not effective for prolonging survival in patients with LAPC.
Trial registration: ClinicalTrials.gov NCT00051467.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Siegel R, Naishadham D, Jemal A: Cancer statistics, 2012 CA Cancer J Clin 62:10–29,2012 - PubMed
-
- Sener SF Fremgen A Menck HR, etal: Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database J Am Coll Surg 189:1–7,1999 - PubMed
-
- Moertel CG Frytak S Hahn RG, etal: Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil—The Gastrointestinal Tumor Study Group Cancer 48:1705–1710,1981 - PubMed
-
- Treatment of locally unresectable carcinoma of the pancreas: Comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone—Gastrointestinal Tumor Study Group J Natl Cancer Inst 80:751–755,1988Gastrointestinal Tumor Study Group - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

