Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo
- PMID: 23341543
- PMCID: PMC3612858
- DOI: 10.1182/blood-2012-12-471201
Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo
Abstract
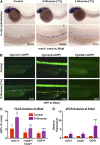
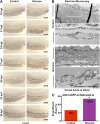
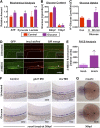
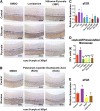
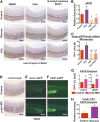
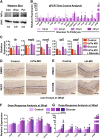
Many pathways regulating blood formation have been elucidated, yet how each coordinates with embryonic biophysiology to modulate the spatiotemporal production of hematopoietic stem cells (HSCs) is currently unresolved. Here, we report that glucose metabolism impacts the onset and magnitude of HSC induction in vivo. In zebrafish, transient elevations in physiological glucose levels elicited dose-dependent effects on HSC development, including enhanced runx1 expression and hematopoietic cluster formation in the aorta-gonad-mesonephros region; embryonic-to-adult transplantation studies confirmed glucose increased functional HSCs. Glucose uptake was required to mediate the enhancement in HSC development; likewise, metabolic inhibitors diminished nascent HSC production and reversed glucose-mediated effects on HSCs. Increased glucose metabolism preferentially impacted hematopoietic and vascular targets, as determined by gene expression analysis, through mitochondrial-derived reactive oxygen species (ROS)-mediated stimulation of hypoxia-inducible factor 1α (hif1α). Epistasis assays demonstrated that hif1α regulates HSC formation in vivo and mediates the dose-dependent effects of glucose metabolism on the timing and magnitude of HSC production. We propose that this fundamental metabolic-sensing mechanism enables the embryo to respond to changes in environmental energy input and adjust hematopoietic output to maintain embryonic growth and ensure viability.
Figures

References
-
- North TE, de Bruijn MF, Stacy T, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16(5):661–672. - PubMed
-
- North T, Gu TL, Stacy T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563–2575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

