Spatial mismatch between the Na+ flux and spike initiation in axon initial segment
- PMID: 23341597
- PMCID: PMC3593864
- DOI: 10.1073/pnas.1215125110
Spatial mismatch between the Na+ flux and spike initiation in axon initial segment
Abstract
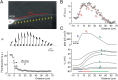
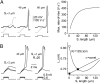
It is widely believed that, in cortical pyramidal cells, action potentials (APs) initiate in the distal portion of axon initial segment (AIS) because that is where Na(+) channel density is highest. To investigate the relationship between the density of Na(+) channels and the spatiotemporal pattern of AP initiation, we simultaneously recorded Na(+) flux and action currents along the proximal axonal length. We found that functional Na(+) channel density is approximately four times lower in the AP trigger zone than in the middle of the AIS, where it is highest. Computational analysis of AP initiation revealed a paradoxical mismatch between the AP threshold and Na(+) channel density, which could be explained by the lopsided capacitive load imposed on the proximal end of the AIS by the somatodendritic compartment. Favorable conditions for AP initiation are therefore achieved in the distal AIS portion, close to the edge of myelin, where the current source-load ratio is highest. Our findings suggest that cable properties play a central role in determining where the AP starts, such that small plastic changes in the local AIS Na(+) channel density could have a large influence on neuronal excitability as a whole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Reemerging role of cable properties in action potential initiation.Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):3715-6. doi: 10.1073/pnas.1300520110. Epub 2013 Feb 25. Proc Natl Acad Sci U S A. 2013. PMID: 23440200 Free PMC article. No abstract available.
References
-
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55(4):633–647. - PubMed
-
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action-potential initiation in neocortical pyramidal cells: Evidence from whole cell axon recordings. J Neurophysiol. 2007;97(1):746–760. - PubMed
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8(6):451–465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

