JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration
- PMID: 23341606
- PMCID: PMC3568328
- DOI: 10.1073/pnas.1221729110
JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration
Abstract
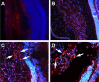
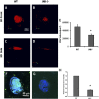
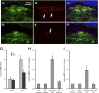
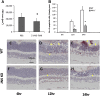
Age-related macular degeneration (AMD) is the leading cause of registered blindness among the elderly and affects over 30 million people worldwide. It is well established that oxidative stress, inflammation, and apoptosis play critical roles in pathogenesis of AMD. In advanced wet AMD, although, most of the severe vision loss is due to bleeding and exudation of choroidal neovascularization (CNV), and it is well known that vascular endothelial growth factor (VEGF) plays a pivotal role in the growth of the abnormal blood vessels. VEGF suppression therapy improves visual acuity in AMD patients. However, there are unresolved issues, including safety and cost. Here we show that mice lacking c-Jun N-terminal kinase 1 (JNK1) exhibit decreased inflammation, reduced CNV, lower levels of choroidal VEGF, and impaired choroidal macrophage recruitment in a murine model of wet AMD (laser-induced CNV). Interestingly, we also detected a substantial reduction in choroidal apoptosis of JNK1-deficient mice. Intravitreal injection of a pan-caspase inhibitor reduced neovascularization in the laser-induced CNV model, suggesting that apoptosis plays a role in laser-induced pathological angiogenesis. Intravitreal injection of a specific JNK inhibitor decreased choroidal VEGF expression and reduced pathological CNV. These results suggest that JNK1 plays a key role in linking oxidative stress, inflammation, macrophage recruitment apoptosis, and VEGF production in wet AMD and pharmacological JNK inhibition offers a unique and alternative avenue for prevention and treatment of AMD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
PEDF expression affects the oxidative and inflammatory state of choroidal endothelial cells.Am J Physiol Cell Physiol. 2018 Apr 1;314(4):C456-C472. doi: 10.1152/ajpcell.00259.2017. Epub 2018 Jan 10. Am J Physiol Cell Physiol. 2018. PMID: 29351407 Free PMC article.
-
RGD-Functionalized Ginsenoside Rg3 Liposomes for Alleviating Oxidative Stress and Choroidal Neovascularization in Age-Related Macular Degeneration.Int J Nanomedicine. 2025 Jun 19;20:7915-7933. doi: 10.2147/IJN.S520756. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40551979 Free PMC article.
-
An efficacy analysis of anti-vascular endothelial growth factor therapy for choroidal neovascularization secondary to multifocal choroiditis and comparison with wet age-related macular degeneration.J Zhejiang Univ Sci B. 2018 Apr.;19(4):327-332. doi: 10.1631/jzus.B1700535. J Zhejiang Univ Sci B. 2018. PMID: 29616508 Free PMC article.
-
Clinical evidence of intravitreal triamcinolone acetonide in the management of age-related macular degeneration.Curr Drug Targets. 2011 Feb;12(2):149-72. doi: 10.2174/138945011794182746. Curr Drug Targets. 2011. PMID: 20887246 Review.
-
Systemic adverse drug reactions secondary to anti-VEGF intravitreal injection in patients with neovascular age-related macular degeneration.Curr Vasc Pharmacol. 2011 Sep;9(5):629-46. doi: 10.2174/157016111796642670. Curr Vasc Pharmacol. 2011. PMID: 21470108 Review.
Cited by
-
Ethanol-Induced Oxidative Stress Modifies Inflammation and Angiogenesis Biomarkers in Retinal Pigment Epithelial Cells (ARPE-19): Role of CYP2E1 and its Inhibition by Antioxidants.Antioxidants (Basel). 2020 Aug 21;9(9):776. doi: 10.3390/antiox9090776. Antioxidants (Basel). 2020. PMID: 32825644 Free PMC article.
-
Apoptotic cell death in disease-Current understanding of the NCCD 2023.Cell Death Differ. 2023 May;30(5):1097-1154. doi: 10.1038/s41418-023-01153-w. Epub 2023 Apr 26. Cell Death Differ. 2023. PMID: 37100955 Free PMC article. Review.
-
Suppression of Age-Related Macular Degeneration-like Pathology by c-Jun N-Terminal Kinase Inhibitor IQ-1S.Biomedicines. 2023 Jan 29;11(2):395. doi: 10.3390/biomedicines11020395. Biomedicines. 2023. PMID: 36830932 Free PMC article.
-
Cytokine profiling reveals increased serum inflammatory cytokines in idiopathic choroidal neovascularization.BMC Ophthalmol. 2019 Apr 24;19(1):94. doi: 10.1186/s12886-019-1101-6. BMC Ophthalmol. 2019. PMID: 31014258 Free PMC article.
-
Metabolic syndrome triggered by high-fructose diet favors choroidal neovascularization and impairs retinal light sensitivity in the rat.PLoS One. 2014 Nov 7;9(11):e112450. doi: 10.1371/journal.pone.0112450. eCollection 2014. PLoS One. 2014. PMID: 25380250 Free PMC article.
References
-
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–2617. - PubMed
-
- Bressler SB. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology. 2009;116(10)(Suppl):S1–S7. - PubMed
-
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28(5):348–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01 EY019270/EY/NEI NIH HHS/United States
- R01 EY014428/EY/NEI NIH HHS/United States
- ES010337/ES/NIEHS NIH HHS/United States
- EY021374/EY/NEI NIH HHS/United States
- R01 ES006376/ES/NIEHS NIH HHS/United States
- EY018660/EY/NEI NIH HHS/United States
- R01 EY018660/EY/NEI NIH HHS/United States
- EY019270/EY/NEI NIH HHS/United States
- EY014428/EY/NEI NIH HHS/United States
- R01 EY021374/EY/NEI NIH HHS/United States
- ES006376/ES/NIEHS NIH HHS/United States
- ES00451/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

