Structures of a Na+-coupled, substrate-bound MATE multidrug transporter
- PMID: 23341609
- PMCID: PMC3568332
- DOI: 10.1073/pnas.1219901110
Structures of a Na+-coupled, substrate-bound MATE multidrug transporter
Abstract
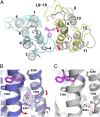
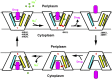
Multidrug transporters belonging to the multidrug and toxic compound extrusion (MATE) family expel dissimilar lipophilic and cationic drugs across cell membranes by dissipating a preexisting Na(+) or H(+) gradient. Despite its clinical relevance, the transport mechanism of MATE proteins remains poorly understood, largely owing to a lack of structural information on the substrate-bound transporter. Here we report crystal structures of a Na(+)-coupled MATE transporter NorM from Neisseria gonorrheae in complexes with three distinct translocation substrates (ethidium, rhodamine 6G, and tetraphenylphosphonium), as well as Cs(+) (a Na(+) congener), all captured in extracellular-facing and drug-bound states. The structures revealed a multidrug-binding cavity festooned with four negatively charged amino acids and surprisingly limited hydrophobic moieties, in stark contrast to the general belief that aromatic amino acids play a prominent role in multidrug recognition. Furthermore, we discovered an uncommon cation-π interaction in the Na(+)-binding site located outside the drug-binding cavity and validated the biological relevance of both the substrate- and cation-binding sites by conducting drug resistance and transport assays. Additionally, we uncovered potential rearrangement of at least two transmembrane helices upon Na(+)-induced drug export. Based on our structural and functional analyses, we suggest that Na(+) triggers multidrug extrusion by inducing protein conformational changes rather than by directly competing for the substrate-binding amino acids. This scenario is distinct from the canonical antiport mechanism, in which both substrate and counterion compete for a shared binding site in the transporter. Collectively, our findings provide an important step toward a detailed and mechanistic understanding of multidrug transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Structure of a cation-bound multidrug and toxic compound extrusion transporter.Nature. 2010 Oct 21;467(7318):991-4. doi: 10.1038/nature09408. Epub 2010 Sep 22. Nature. 2010. PMID: 20861838 Free PMC article.
-
Sodium and proton coupling in the conformational cycle of a MATE antiporter from Vibrio cholerae.Proc Natl Acad Sci U S A. 2018 Jul 3;115(27):E6182-E6190. doi: 10.1073/pnas.1802417115. Epub 2018 Jun 18. Proc Natl Acad Sci U S A. 2018. PMID: 29915043 Free PMC article.
-
Insights on Na(+) binding and conformational dynamics in multidrug and toxic compound extrusion transporter NorM.Proteins. 2014 Feb;82(2):240-9. doi: 10.1002/prot.24368. Epub 2013 Sep 17. Proteins. 2014. PMID: 23873591
-
Structures of multidrug and toxic compound extrusion transporters and their mechanistic implications.Channels (Austin). 2016;10(2):88-100. doi: 10.1080/19336950.2015.1106654. Epub 2015 Oct 21. Channels (Austin). 2016. PMID: 26488689 Free PMC article. Review.
-
Molecular properties of bacterial multidrug transporters.Microbiol Mol Biol Rev. 2000 Dec;64(4):672-93. doi: 10.1128/MMBR.64.4.672-693.2000. Microbiol Mol Biol Rev. 2000. PMID: 11104814 Free PMC article. Review.
Cited by
-
Automatic Inference of Sequence from Low-Resolution Crystallographic Data.Structure. 2018 Nov 6;26(11):1546-1554.e2. doi: 10.1016/j.str.2018.08.011. Epub 2018 Oct 4. Structure. 2018. PMID: 30293812 Free PMC article.
-
Inward-facing conformation of a multidrug resistance MATE family transporter.Proc Natl Acad Sci U S A. 2019 Jun 18;116(25):12275-12284. doi: 10.1073/pnas.1904210116. Epub 2019 Jun 3. Proc Natl Acad Sci U S A. 2019. PMID: 31160466 Free PMC article.
-
Engineered MATE multidrug transporters reveal two functionally distinct ion-coupling pathways in NorM from Vibrio cholerae.Commun Biol. 2021 May 11;4(1):558. doi: 10.1038/s42003-021-02081-6. Commun Biol. 2021. PMID: 33976372 Free PMC article.
-
Prediction of protein structural changes mediated by NS-SNPs in antibiotic resistance determinants in Streptococcus pneumoniae.Arch Microbiol. 2025 Aug 29;207(10):243. doi: 10.1007/s00203-025-04444-7. Arch Microbiol. 2025. PMID: 40879807
-
Structure, Assembly, and Function of Tripartite Efflux and Type 1 Secretion Systems in Gram-Negative Bacteria.Chem Rev. 2021 May 12;121(9):5479-5596. doi: 10.1021/acs.chemrev.1c00055. Epub 2021 Apr 28. Chem Rev. 2021. PMID: 33909410 Free PMC article. Review.
References
-
- Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446(7137):749–757. - PubMed
-
- Brown MH, Paulsen IT, Skurray RA. The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol. 1999;31(1):394–395. - PubMed
-
- Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci. 2006;27(11):587–593. - PubMed
-
- Kuroda T, Tsuchiya T. Multidrug efflux transporters in the MATE family. Biochim Biophys Acta. 2009;1794(5):763–768. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

