Dependence receptor TrkC is a putative colon cancer tumor suppressor
- PMID: 23341610
- PMCID: PMC3581924
- DOI: 10.1073/pnas.1212333110
Dependence receptor TrkC is a putative colon cancer tumor suppressor
Abstract
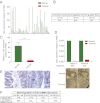
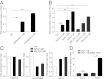
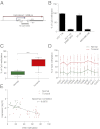
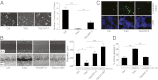
The TrkC neurotrophin receptor belongs to the functional dependence receptor family, members of which share the ability to induce apoptosis in the absence of their ligands. Such a trait has been hypothesized to confer tumor-suppressor activity. Indeed, cells that express these receptors are thought to be dependent on ligand availability for their survival, a mechanism that inhibits uncontrolled tumor cell proliferation and migration. TrkC is a classic tyrosine kinase receptor and therefore generally considered to be a proto-oncogene. We show here that TrkC expression is down-regulated in a large fraction of human colorectal cancers, mainly through promoter methylation. Moreover, we show that TrkC silencing by promoter methylation is a selective advantage for colorectal cell lines to limit tumor cell death. Furthermore, reestablished TrkC expression in colorectal cancer cell lines is associated with tumor cell death and inhibition of in vitro characteristics of cell transformation, as well as in vivo tumor growth. Finally, we provide evidence that a mutation of TrkC detected in a sporadic cancer is a loss-of-proapoptotic function mutation. Together, these data support the conclusion that TrkC is a colorectal cancer tumor suppressor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Context is everything for dependence receptors in colorectal cancer.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):2697-8. doi: 10.1073/pnas.1300758110. Epub 2013 Feb 8. Proc Natl Acad Sci U S A. 2013. PMID: 23396845 Free PMC article. No abstract available.
Similar articles
-
NTRK3 is a potential tumor suppressor gene commonly inactivated by epigenetic mechanisms in colorectal cancer.PLoS Genet. 2013;9(7):e1003552. doi: 10.1371/journal.pgen.1003552. Epub 2013 Jul 11. PLoS Genet. 2013. PMID: 23874207 Free PMC article.
-
Inactivation of the UNC5C Netrin-1 receptor is associated with tumor progression in colorectal malignancies.Gastroenterology. 2007 Dec;133(6):1840-8. doi: 10.1053/j.gastro.2007.08.009. Epub 2007 Aug 2. Gastroenterology. 2007. PMID: 17967459 Free PMC article.
-
Targeting neurotrophin-3 and its dependence receptor tyrosine kinase receptor C: a new antitumoral strategy.Expert Opin Ther Targets. 2011 Jul;15(7):847-58. doi: 10.1517/14728222.2011.575361. Epub 2011 Apr 8. Expert Opin Ther Targets. 2011. PMID: 21473736 Review.
-
Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis.J Clin Invest. 2010 Mar;120(3):850-8. doi: 10.1172/JCI41013. Epub 2010 Feb 15. J Clin Invest. 2010. PMID: 20160348 Free PMC article.
-
BDNF: An Oncogene or Tumor Suppressor?Anticancer Res. 2017 Aug;37(8):3983-3990. doi: 10.21873/anticanres.11783. Anticancer Res. 2017. PMID: 28739680 Review.
Cited by
-
Asparaginase treatment side-effects may be due to genes with homopolymeric Asn codons (Review-Hypothesis).Int J Mol Med. 2015 Sep;36(3):607-26. doi: 10.3892/ijmm.2015.2285. Epub 2015 Jul 15. Int J Mol Med. 2015. PMID: 26178806 Free PMC article. Review.
-
Emerging Roles of the Nervous System in Gastrointestinal Cancer Development.Cancers (Basel). 2022 Jul 30;14(15):3722. doi: 10.3390/cancers14153722. Cancers (Basel). 2022. PMID: 35954387 Free PMC article. Review.
-
TrkC promotes colorectal cancer growth and metastasis.Oncotarget. 2017 Jun 20;8(25):41319-41333. doi: 10.18632/oncotarget.17289. Oncotarget. 2017. PMID: 28455963 Free PMC article.
-
Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018.Cell Death Differ. 2018 Mar;25(3):486-541. doi: 10.1038/s41418-017-0012-4. Epub 2018 Jan 23. Cell Death Differ. 2018. PMID: 29362479 Free PMC article. Review.
-
Modifying PCDH19 levels affects cortical interneuron migration.Front Neurosci. 2022 Oct 25;16:887478. doi: 10.3389/fnins.2022.887478. eCollection 2022. Front Neurosci. 2022. PMID: 36389226 Free PMC article.
References
-
- Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319(6056):743–748. - PubMed
-
- Nakagawara A. Trk receptor tyrosine kinases: A bridge between cancer and neural development. Cancer Lett. 2001;169(2):107–114. - PubMed
-
- Marshall JL, et al. Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs. 2005;23(1):31–37. - PubMed
-
- Knapper S, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–3270. - PubMed
-
- Chan E, et al. A phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008;26(3):241–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

