Structure of ADP-aluminium fluoride-stabilized protochlorophyllide oxidoreductase complex
- PMID: 23341615
- PMCID: PMC3568340
- DOI: 10.1073/pnas.1218303110
Structure of ADP-aluminium fluoride-stabilized protochlorophyllide oxidoreductase complex
Abstract
Photosynthesis uses chlorophylls for the conversion of light into chemical energy, the driving force of life on Earth. During chlorophyll biosynthesis in photosynthetic bacteria, cyanobacteria, green algae and gymnosperms, dark-operative protochlorophyllide oxidoreductase (DPOR), a nitrogenase-like metalloenzyme, catalyzes the chemically challenging two-electron reduction of the fully conjugated ring system of protochlorophyllide a. The reduction of the C-17=C-18 double bond results in the characteristic ring architecture of all chlorophylls, thereby altering the absorption properties of the molecule and providing the basis for light-capturing and energy-transduction processes of photosynthesis. We report the X-ray crystallographic structure of the substrate-bound, ADP-aluminium fluoride-stabilized (ADP·AlF(3)-stabilized) transition state complex between the DPOR components L(2) and (NB)(2) from the marine cyanobacterium Prochlorococcus marinus. Our analysis permits a thorough investigation of the dynamic interplay between L(2) and (NB)(2). Upon complex formation, substantial ATP-dependent conformational rearrangements of L(2) trigger the protein-protein interactions with (NB)(2) as well as the electron transduction via redox-active [4Fe-4S] clusters. We also present the identification of artificial "small-molecule substrates" of DPOR in correlation with those of nitrogenase. The catalytic differences and similarities between DPOR and nitrogenase have broad implications for the energy transduction mechanism of related multiprotein complexes that are involved in the reduction of chemically stable double and/or triple bonds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

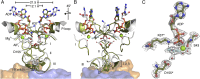
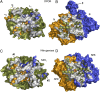
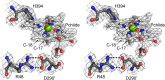
Similar articles
-
Evolution of light-independent protochlorophyllide oxidoreductase.Protoplasma. 2019 Mar;256(2):293-312. doi: 10.1007/s00709-018-1317-y. Epub 2018 Oct 6. Protoplasma. 2019. PMID: 30291443 Review.
-
Substrate recognition of nitrogenase-like dark operative protochlorophyllide oxidoreductase from Prochlorococcus marinus.J Biol Chem. 2008 Oct 31;283(44):29873-81. doi: 10.1074/jbc.M805206200. Epub 2008 Aug 8. J Biol Chem. 2008. PMID: 18693243 Free PMC article.
-
Biosynthesis of (bacterio)chlorophylls: ATP-dependent transient subunit interaction and electron transfer of dark operative protochlorophyllide oxidoreductase.J Biol Chem. 2010 Mar 12;285(11):8268-77. doi: 10.1074/jbc.M109.087874. Epub 2010 Jan 14. J Biol Chem. 2010. PMID: 20075073 Free PMC article.
-
Reduction of Chemically Stable Multibonds: Nitrogenase-Like Biosynthesis of Tetrapyrroles.Adv Exp Med Biol. 2017;925:147-161. doi: 10.1007/5584_2016_175. Adv Exp Med Biol. 2017. PMID: 27957709 Review.
-
Methods for nitrogenase-like dark operative protochlorophyllide oxidoreductase.Methods Mol Biol. 2011;766:129-43. doi: 10.1007/978-1-61779-194-9_9. Methods Mol Biol. 2011. PMID: 21833865
Cited by
-
Nitrogenase and homologs.J Biol Inorg Chem. 2015 Mar;20(2):435-45. doi: 10.1007/s00775-014-1225-3. Epub 2014 Dec 10. J Biol Inorg Chem. 2015. PMID: 25491285 Free PMC article. Review.
-
Substrate recognition induces sequential electron transfer across subunits in the nitrogenase-like DPOR complex.J Biol Chem. 2020 Sep 25;295(39):13630-13639. doi: 10.1074/jbc.RA120.015151. Epub 2020 Jul 31. J Biol Chem. 2020. PMID: 32737200 Free PMC article.
-
Evolution of light-independent protochlorophyllide oxidoreductase.Protoplasma. 2019 Mar;256(2):293-312. doi: 10.1007/s00709-018-1317-y. Epub 2018 Oct 6. Protoplasma. 2019. PMID: 30291443 Review.
-
Iron-sulfur cluster-dependent catalysis of chlorophyllide a oxidoreductase from Roseobacter denitrificans.J Biol Chem. 2015 Jan 9;290(2):1141-54. doi: 10.1074/jbc.M114.617761. Epub 2014 Nov 24. J Biol Chem. 2015. PMID: 25422320 Free PMC article.
-
Dark-operative protochlorophyllide oxidoreductase generates substrate radicals by an iron-sulphur cluster in bacteriochlorophyll biosynthesis.Sci Rep. 2014 Jun 26;4:5455. doi: 10.1038/srep05455. Sci Rep. 2014. PMID: 24965831 Free PMC article.
References
-
- Heyes DJ, Hunter CN. Making light work of enzyme catalysis: Protochlorophyllide oxidoreductase. Trends Biochem Sci. 2005;30(11):642–649. - PubMed
-
- Fujita Y. Protochlorophyllide reduction: A key step in the greening of plants. Plant Cell Physiol. 1996;37(4):411–421. - PubMed
-
- Fujita Y, Bauer CE. Reconstitution of light-independent protochlorophyllide reductase from purified bchl and BchN-BchB subunits. In vitro confirmation of nitrogenase-like features of a bacteriochlorophyll biosynthesis enzyme. J Biol Chem. 2000;275(31):23583–23588. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

