Fas expression by tumor stroma is required for cancer eradication
- PMID: 23341634
- PMCID: PMC3568383
- DOI: 10.1073/pnas.1218295110
Fas expression by tumor stroma is required for cancer eradication
Abstract
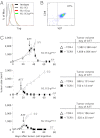
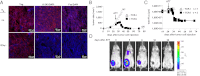
The contribution of molecules such as perforin, IFN-γ (IFNγ), and particularly Fas ligand (FasL) by transferred CD8(+) effector T (T(E)) cells to rejection of large, established tumors is incompletely understood. Efficient attack against large tumors carrying a surrogate tumor antigen (mimicking a "passenger" mutation) by T(E) cells requires action of IFNγ on tumor stroma cells to avoid selection of antigen-loss variants. Because "cancer-driving" antigens (CDAs) are rarely counterselected, IFNγ may be expected to be dispensable in elimination of cancers by targeting a CDA. Here, initial regression of large, established tumors required neither IFNγ, FasL, nor perforin by transferred CD8(+) T(E) cells targeting Simian Virus (SV) 40 large T as CDA. However, cytotoxic T(E) cells lacking IFNγ or FasL could not prevent relapse despite retention of the rejection antigen by the cancer cells. Complete tumor rejection required IFNγ-regulated Fas by the tumor stroma. Therefore, T(E) cells lacking IFNγ or FasL cannot prevent progression of antigenic cancer because the tumor stroma escapes destruction if its Fas expression is down-regulated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zippelius A, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: A state of local functional tolerance. Cancer Res. 2004;64(8):2865–2873. - PubMed
-
- Schreiber K, Rowley DA, Riethmüller G, Schreiber H. Cancer immunotherapy and preclinical studies: Why we are not wasting our time with animal experiments. Hematol Oncol Clin North Am. 2006;20(3):567–584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

