Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement
- PMID: 23341635
- PMCID: PMC3568385
- DOI: 10.1073/pnas.1218933110
Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement
Abstract
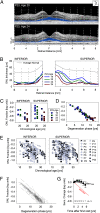
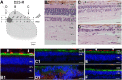
Leber congenital amaurosis (LCA) associated with retinal pigment epithelium-specific protein 65 kDa (RPE65) mutations is a severe hereditary blindness resulting from both dysfunction and degeneration of photoreceptors. Clinical trials with gene augmentation therapy have shown partial reversal of the dysfunction, but the effects on the degeneration are not known. We evaluated the consequences of gene therapy on retinal degeneration in patients with RPE65-LCA and its canine model. In untreated RPE65-LCA patients, there was dysfunction and degeneration of photoreceptors, even at the earliest ages. Examined serially over years, the outer photoreceptor nuclear layer showed progressive thinning. Treated RPE65-LCA showed substantial visual improvement in the short term and no detectable decline from this new level over the long term. However, retinal degeneration continued to progress unabated. In RPE65-mutant dogs, the first one-quarter of their lifespan showed only dysfunction, and there was normal outer photoreceptor nuclear layer thickness retina-wide. Dogs treated during the earlier dysfunction-only stage showed improved visual function and dramatic protection of treated photoreceptors from degeneration when measured 5-11 y later. Dogs treated later during the combined dysfunction and degeneration stage also showed visual function improvement, but photoreceptor loss continued unabated, the same as in human RPE65-LCA. The results suggest that, in RPE65 disease treatment, protection from visual function deterioration cannot be assumed to imply protection from degeneration. The effects of gene augmentation therapy are complex and suggest a need for a combinatorial strategy in RPE65-LCA to not only improve function in the short term but also slow retinal degeneration in the long term.
Conflict of interest statement
Conflict of interest statement: W.W.H. and the University of Florida have a financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. The remaining authors declare no conflict of interest. University of Pennsylvania, University of Florida, and Cornell University hold a patent on the described gene therapy technology (United States Patent 20070077228, “Method for Treating or Retarding the Development of Blindness”).
Figures



Comment in
-
Retinal gene therapy coming of age.Hum Gene Ther. 2013 Mar;24(3):242-4. doi: 10.1089/hum.2013.050. Hum Gene Ther. 2013. PMID: 23458444 Free PMC article. No abstract available.
-
Increased levels of gene therapy may not be beneficial in retinal disease.Proc Natl Acad Sci U S A. 2013 May 7;110(19):E1705. doi: 10.1073/pnas.1303746110. Epub 2013 Apr 3. Proc Natl Acad Sci U S A. 2013. PMID: 23553840 Free PMC article. No abstract available.
-
Reply to Townes-Anderson: RPE65 gene therapy does not alter the natural history of retinal degeneration.Proc Natl Acad Sci U S A. 2013 May 7;110(19):E1706. doi: 10.1073/pnas.1304296110. Proc Natl Acad Sci U S A. 2013. PMID: 23789127 Free PMC article. No abstract available.
References
-
- Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–472. - PubMed
-
- Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. - PubMed
-
- Aguirre GD, et al. Congenital stationary night blindness in the dog: Common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

