HSV carrying WT REST establishes latency but reactivates only if the synthesis of REST is suppressed
- PMID: 23341636
- PMCID: PMC3568314
- DOI: 10.1073/pnas.1222497110
HSV carrying WT REST establishes latency but reactivates only if the synthesis of REST is suppressed
Abstract
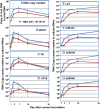
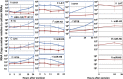
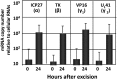
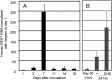
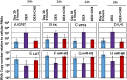
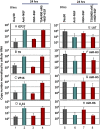
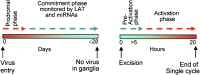
HSVs transit from vigorous replication at the portal of entry into the body to a latent state in sensory neurons in which only noncoding (e.g., latency-associated transcript) and micro-RNAs are expressed. In productive infection, viral genes must be sequentially derepressed at two checkpoints. A leading role in the repression of viral genes is carried out by histone deacetylase (HDAC)/corepressor element-1 silencing transcription factor (CoREST)/lysinespecific demethylase1(LSD1)/RE1-silencing transcription factor (REST) repressor complex (HCLR). Previously, we reported that to define the role of the components of the HCLR complex in the establishment of latency, we constructed recombinant virus (R112) carrying a dominant-negative REST that bound response elements in DNA but could not recruit repressive proteins. This recombinant virus was unable to establish latency. In the current studies, we constructed a virus (R111) carrying WT REST with a WT genome. We report the following findings: (a) R111 readily established latent infection in trigeminal ganglia; however, although the amounts of viral DNAs in latently infected neurons were similar to those of WT virus, the levels of latency-associated transcript and micro-RNAs were 50- to 100-fold lower; (b) R111 did not spontaneously reactivate in ganglionic organ cultures; however, viral genes were expressed if the synthesis of REST was blocked by cycloheximide; and (c) histone deacetylase inhibitors reactivated the WT parent but not the R111 recombinant virus. The results suggest that REST plays a transient role in the establishment of latency but not in reactivation and suggest the existence of at least two phases at both establishment and reactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
HSV-1 gene expression from reactivated ganglia is disordered and concurrent with suppression of latency-associated transcript and miRNAs.Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18820-4. doi: 10.1073/pnas.1117203108. Epub 2011 Nov 7. Proc Natl Acad Sci U S A. 2011. PMID: 22065742 Free PMC article.
-
Induction of apoptosis accelerates reactivation of latent HSV-1 in ganglionic organ cultures and replication in cell cultures.Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14616-21. doi: 10.1073/pnas.1212661109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908263 Free PMC article.
-
The role of the CoREST/REST repressor complex in herpes simplex virus 1 productive infection and in latency.Viruses. 2013 Apr 29;5(5):1208-18. doi: 10.3390/v5051208. Viruses. 2013. PMID: 23628827 Free PMC article. Review.
-
Mutations Inactivating Herpes Simplex Virus 1 MicroRNA miR-H2 Do Not Detectably Increase ICP0 Gene Expression in Infected Cultured Cells or Mouse Trigeminal Ganglia.J Virol. 2017 Jan 3;91(2):e02001-16. doi: 10.1128/JVI.02001-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847363 Free PMC article.
-
Checkpoints in productive and latent infections with herpes simplex virus 1: conceptualization of the issues.J Neurovirol. 2011 Dec;17(6):512-7. doi: 10.1007/s13365-011-0058-x. Epub 2011 Nov 4. J Neurovirol. 2011. PMID: 22052379 Review.
Cited by
-
Role of ND10 nuclear bodies in the chromatin repression of HSV-1.Virol J. 2016 Apr 5;13:62. doi: 10.1186/s12985-016-0516-4. Virol J. 2016. PMID: 27048561 Free PMC article. Review.
-
Modulation of reactivation of latent herpes simplex virus 1 in ganglionic organ cultures by p300/CBP and STAT3.Proc Natl Acad Sci U S A. 2013 Jul 9;110(28):E2621-8. doi: 10.1073/pnas.1309906110. Epub 2013 Jun 20. Proc Natl Acad Sci U S A. 2013. PMID: 23788661 Free PMC article.
-
Chromatin dynamics during lytic infection with herpes simplex virus 1.Viruses. 2013 Jul 16;5(7):1758-86. doi: 10.3390/v5071758. Viruses. 2013. PMID: 23863878 Free PMC article. Review.
-
Differentially expressed genes during spontaneous lytic switch of Marek's disease virus in lymphoblastoid cell lines determined by global gene expression profiling.J Gen Virol. 2017 Apr;98(4):779-790. doi: 10.1099/jgv.0.000744. J Gen Virol. 2017. PMID: 28475033 Free PMC article.
-
Intrinsic and Innate Defenses of Neurons: Détente with the Herpesviruses.J Virol. 2016 Dec 16;91(1):e01200-16. doi: 10.1128/JVI.01200-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795407 Free PMC article. Review.
References
-
- Roizman B, Zhou G, Du T. Checkpoints in productive and latent infections with herpes simplex virus 1: Conceptualization of the issues. J Neurovirol. 2011;17(6):512–517. - PubMed
-
- Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6th Ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

