Identifying transmission cycles at the human-animal interface: the role of animal reservoirs in maintaining gambiense human african trypanosomiasis
- PMID: 23341760
- PMCID: PMC3547827
- DOI: 10.1371/journal.pcbi.1002855
Identifying transmission cycles at the human-animal interface: the role of animal reservoirs in maintaining gambiense human african trypanosomiasis
Abstract
Many infections can be transmitted between animals and humans. The epidemiological roles of different species can vary from important reservoirs to dead-end hosts. Here, we present a method to identify transmission cycles in different combinations of species from field data. We used this method to synthesise epidemiological and ecological data from Bipindi, Cameroon, a historical focus of gambiense Human African Trypanosomiasis (HAT, sleeping sickness), a disease that has often been considered to be maintained mainly by humans. We estimated the basic reproduction number [Formula: see text] of gambiense HAT in Bipindi and evaluated the potential for transmission in the absence of human cases. We found that under the assumption of random mixing between vectors and hosts, gambiense HAT could not be maintained in this focus without the contribution of animals. This result remains robust under extensive sensitivity analysis. When using the distributions of species among habitats to estimate the amount of mixing between those species, we found indications for an independent transmission cycle in wild animals. Stochastic simulation of the system confirmed that unless vectors moved between species very rarely, reintroduction would usually occur shortly after elimination of the infection from human populations. This suggests that elimination strategies may have to be reconsidered as targeting human cases alone would be insufficient for control, and reintroduction from animal reservoirs would remain a threat. Our approach is broadly applicable and could reveal animal reservoirs critical to the control of other infectious diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

 under the assumption of random mixing between vectors and hosts. (b) The contribution of different sets of species to
under the assumption of random mixing between vectors and hosts. (b) The contribution of different sets of species to  under the assumption of random mixing between vectors and hosts. In both plots, the y-axis shows the values of
under the assumption of random mixing between vectors and hosts. In both plots, the y-axis shows the values of  which would be found in a system of only the given (set of) species and vectors, the central line indicating the most likely value, upper and lower edges the interquartile range, the outer lines 1.5 times the interquartile range, and individual dots outlier results. The rightmost data point in (b) shows the estimate for
which would be found in a system of only the given (set of) species and vectors, the central line indicating the most likely value, upper and lower edges the interquartile range, the outer lines 1.5 times the interquartile range, and individual dots outlier results. The rightmost data point in (b) shows the estimate for  in the whole system (all species combined). Outliers for white-eyelid mangabeys with
in the whole system (all species combined). Outliers for white-eyelid mangabeys with  (0.1% of values) are not shown.
(0.1% of values) are not shown.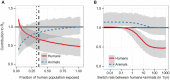
 as a function of the fraction of the population exposed to bites of the vector, shown here as effective population size
as a function of the fraction of the population exposed to bites of the vector, shown here as effective population size  . The vertical dashed line indicates the fraction of the population in the main endemic area , and the dotted line 90% of that population, a low estimate for screening efficacy . (b) The contribution of the human (red, solid) and animal (blue, dashed) populations to
. The vertical dashed line indicates the fraction of the population in the main endemic area , and the dotted line 90% of that population, a low estimate for screening efficacy . (b) The contribution of the human (red, solid) and animal (blue, dashed) populations to  as a function of the rate of host switching between a species, given in units of (number of switches)/year/fly. In both plots, the y-axis shows the values of
as a function of the rate of host switching between a species, given in units of (number of switches)/year/fly. In both plots, the y-axis shows the values of  which would be found in a system of only humans and the vector. The lines show the best estimate, and the light grey areas contain the smoothed (2.5%, 97.5%) quantile range, obtained from the binomial likelihood profiles and Latin hypercube sampling of parameter ranges (see Supporting Text S2).
which would be found in a system of only humans and the vector. The lines show the best estimate, and the light grey areas contain the smoothed (2.5%, 97.5%) quantile range, obtained from the binomial likelihood profiles and Latin hypercube sampling of parameter ranges (see Supporting Text S2).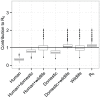
 under the assumption of mixing proportional to habitat overlap of hosts. Hosts are grouped according to the habitats they can be found in, with random mixing within these groups and switching occurring at a third of the biting rate between the groups. The y-axis shows the values of
under the assumption of mixing proportional to habitat overlap of hosts. Hosts are grouped according to the habitats they can be found in, with random mixing within these groups and switching occurring at a third of the biting rate between the groups. The y-axis shows the values of  which would be found in a system of only the given set of species and vectors, the central line indicating the most likely value, upper and lower edges the interquartile range, the outer lines 1.5 times the interquartile range, and individual dots outlier results. The rightmost data point in shows the estimate for
which would be found in a system of only the given set of species and vectors, the central line indicating the most likely value, upper and lower edges the interquartile range, the outer lines 1.5 times the interquartile range, and individual dots outlier results. The rightmost data point in shows the estimate for  in the whole system (all species combined).
in the whole system (all species combined).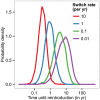
 stochastic simulations, initialised with the prevalence in animal populations as measured in Bipindi, but with no infection present in humans, domestic animals, or human-associated vectors. Simulations were initialised with
stochastic simulations, initialised with the prevalence in animal populations as measured in Bipindi, but with no infection present in humans, domestic animals, or human-associated vectors. Simulations were initialised with  vectors, based on the number of around 2,000–3,000 flies captured in the area through entomological surveys lasting a few days . We considered reintroduction to have occurred once there were 2 cases in humans at any given time.
vectors, based on the number of around 2,000–3,000 flies captured in the area through entomological surveys lasting a few days . We considered reintroduction to have occurred once there were 2 cases in humans at any given time.References
-
- Mulligan HW, Potts WH (1970) The African trypanosomiases. Hoboken: Wiley. 950 pp.
-
- Hoare CA (1972) The trypanosomes of mammals. A zoological monograph. Oxford: Blackwell. 749 pp.
-
- Leak SGA (1999) Tsetse Biology and Ecology: Their Role in the Epidemiology and Control of Trypanosmosis. Wallingford: CABI. 448 pp.
-
- Maudlin I, Holmes PH, Miles MA (2004) The Trypanosomiases. Wallingford: CABI.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

