ADAM17 mediates MMP9 expression in lung epithelial cells
- PMID: 23341882
- PMCID: PMC3544892
- DOI: 10.1371/journal.pone.0051701
ADAM17 mediates MMP9 expression in lung epithelial cells
Abstract
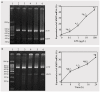
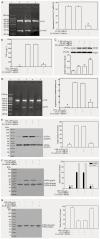
The purposes were to study the role of lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α/nuclear factor-κB (NF-κB) signaling in matrix metalloproteinase 9 (MMP9) expression in A549 cells and to investigate the effects of lentivirus-mediated RNAi targeting of the disintegrin and metalloproteinase 17 (ADAM17) gene on LPS-induced MMP9 expression. MMP9 expression induced by LPS in A549 cells was significantly increased in a dose- and time-dependent manner (p<0.05). Pyrrolidine dithiocarbamate (PDTC) and a TNFR1 blocking peptide (TNFR1BP) significantly inhibited LPS-induced MMP9 expression in A549 cells (p<0.05). TNFR1BP significantly inhibited LPS-induced TNF-α production (p<0.05). Both PDTC and TNFR1BP significantly inhibited the phosphorylation of IκBα and expression of phosphorylation p65 protein in response to LPS (p<0.05), and the level of IκBα in the cytoplasm was significantly increased (p<0.05). Lentivirus mediated RNA interference (RNAi) significantly inhibited ADAM17 expression in A549 cells. Lentivirus-mediated RNAi targeting of ADAM17 significantly inhibited TNF-α production in the supernatants (p<0.05), whereas the level of TNF-α in the cells was increased (p<0.05). Lentiviral ADAM17 RNAi inhibited MMP9 expression, IκBα phosphorylation and the expression of phosphorylation p65 protein in response to LPS (p<0.05). PDTC significantly inhibited the expression of MMP9 and the phosphorylation of IκBα, as well as the expression of phosphorylation p65 protein in response to TNF-α (p<0.05). Lentiviral RNAi targeting of ADAM17 down-regulates LPS-induced MMP9 expression in lung epithelial cells via inhibition of TNF-α/NF-κB signaling.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

