Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis
- PMID: 23341902
- PMCID: PMC3541389
- DOI: 10.1371/journal.pone.0052562
Clinical use of HIV integrase inhibitors: a systematic review and meta-analysis
Abstract
Background: Optimal regimen choice of antiretroviral therapy is essential to achieve long-term clinical success. Integrase inhibitors have swiftly been adopted as part of current antiretroviral regimens. The purpose of this study was to review the evidence for integrase inhibitor use in clinical settings.
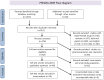
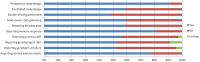
Methods: MEDLINE and Web-of-Science were screened from April 2006 until November 2012, as were hand-searched scientific meeting proceedings. Multiple reviewers independently screened 1323 citations in duplicate to identify randomized controlled trials, nonrandomized controlled trials and cohort studies on integrase inhibitor use in clinical practice. Independent, duplicate data extraction and quality assessment were conducted.
Results: 48 unique studies were included on the use of integrase inhibitors in antiretroviral therapy-naive patients and treatment-experienced patients with either virological failure or switching to integrase inhibitors while virologically suppressed. On the selected studies with comparable outcome measures and indication (n = 16), a meta-analysis was performed based on modified intention-to-treat (mITT), on-treatment (OT) and as-treated (AT) virological outcome data. In therapy-naive patients, favorable odds ratios (OR) for integrase inhibitor-based regimens were observed, (mITT OR 0.71, 95% CI 0.59-0.86). However, integrase inhibitors combined with protease inhibitors only did not result in a significant better virological outcome. Evidence further supported integrase inhibitor use following virological failure (mITT OR 0.27; 95% CI 0.11-0.66), but switching to integrase inhibitors from a high genetic barrier drug during successful treatment was not supported (mITT OR 1.43; 95% CI 0.89-2.31). Integrase inhibitor-based regimens result in similar immunological responses compared to other regimens. A low genetic barrier to drug-resistance development was observed for raltegravir and elvitegravir, but not for dolutegravir.
Conclusion: In first-line therapy, integrase inhibitors are superior to other regimens. Integrase inhibitor use after virological failure is supported as well by the meta-analysis. Careful use is however warranted when replacing a high genetic barrier drug in treatment-experienced patients switching successful treatment.
Conflict of interest statement
Figures

References
-
- Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. Accessible at http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_.... Accessed 16/10/2012 2012/10/16. Geneva.
-
- Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, et al. (2008) Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 359: 339–354. - PubMed
-
- Yazdanpanah Y, Fagard C, Descamps D, Taburet AM, Colin C, et al. (2009) High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis 49: 1441–1449. - PubMed
-
- Department of Health and Human Services. DHHS Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents - October 14, 2011. Accessible at http://www.aidsinfo.nih.gov/Guidelines/GuidelineDetail.aspx?GuidelineID=7. Accessed 2012/10/16.
-
- European AIDS Clinical Society. EACS European Guidelines for treatment of HIV infected adults in Europe. Accessible at http://www.europeanaidsclinicalsociety.org/index.php?option=com_content&.... Accessed 2012/10/16.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

