The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2
- PMID: 23341968
- PMCID: PMC3544925
- DOI: 10.1371/journal.pone.0053662
The protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2
Abstract
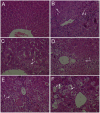
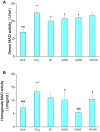
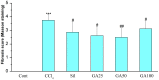
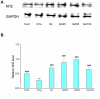
This study was designed to investigate the potentially protective effects of glycyrrhetinic acid (GA) and the role of transcription factor nuclear factor-erythroid 2(NF-E2)-related factor 2 (Nrf2) signaling in the regulation of Carbon Tetrachloride (CCl(4))-induced chronic liver fibrosis in mice. The potentially protective effects of GA on CCl(4)-induced chronic liver fibrosis in mice were depicted histologically and biochemically. Firstly, histopathological changes including regenerative nodules, inflammatory cell infiltration and fibrosis were induced by CCl(4).Then, CCl(4) administration caused a marked increase in the levels of serum aminotransferases (GOT, GPT), serum monoamine oxidase (MAO) and lipid peroxidation (MDA) as well as MAO in the mice liver homogenates. Also, decreased nuclear Nrf2 expression, mRNA levels of its target genes such as superoxide dismutase 3 (SOD3), catalase (CAT), glutathione peroxidase 2 (GPX2), and activity of cellular antioxidant enzymes were found after CCl(4) exposure. All of these phenotypes were markedly reversed by the treatment of the mice with GA. In addition, GA exhibited the antioxidant effects in vitro by on FeCl(2)-ascorbate induced lipid peroxidation in mouse liver homogenates, and on DPPH scavenging activity. Taken together, these results suggested that GA can protect the liver from oxidative stress in mice, presumably through activating the nuclear translocation of Nrf2, enhancing the expression of its target genes and increasing the activity of the antioxidant enzymes. Therefore, GA may be an effective hepatoprotective agent and viable candidate for treating liver fibrosis and other oxidative stress-related diseases.
Conflict of interest statement
Figures







References
-
- Kim HK, Li L, Lee HS, Park MO, Bilehal D, et al. (2009) Protective effects of Chlorella vulgaris Extract on carbon tetrachloride-induced acute liver injury in mice. Food Sci Biotechnol 18: 1186–1192.
-
- Guyton AC, Hall JE (2006) The liver as an organ. In: Guyton AC, Hall JE editors. Textbook of Medical Physiology. 11th ed. Philadelphia: Saunders Elsevier. 859–864.
-
- Friedman SL (2003) Hepatic fibrosis. In: Schiff ER, Sorrell MF, Maddrey WC editors. Schiff’s Diseases of the Liver. 9th ed. Philadelphia: Lippinocott Williams & Wilkins. 409–427.
-
- Poli G (2000) Pathogenesis of liverfibrosis: role of oxidative stress. Mol Aspects Med 21: 49–98. - PubMed
-
- Ivanov AV, Smirnova OA, Ivanova ON, Masalova OV, Kochetkov SN, et al. (2011) Hepatitis C Virus Proteins Activate NRF2/ARE Pathway by Distinct ROS Dependent and Independent Mechanisms in HUH7 Cells. PLoS ONE 6(9): e24957 doi:10.1371/journal.pone.0024957. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

