Gene expression deregulation in postnatal skeletal muscle of TK2 deficient mice reveals a lower pool of proliferating myogenic progenitor cells
- PMID: 23341978
- PMCID: PMC3544874
- DOI: 10.1371/journal.pone.0053698
Gene expression deregulation in postnatal skeletal muscle of TK2 deficient mice reveals a lower pool of proliferating myogenic progenitor cells
Abstract
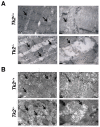
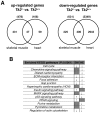
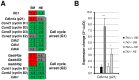
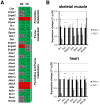
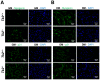
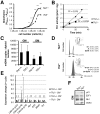
Loss of thymidine kinase 2 (TK2) causes a heterogeneous myopathic form of mitochondrial DNA (mtDNA) depletion syndrome (MDS) in humans that predominantly affects skeletal muscle tissue. In mice, TK2 deficiency also affects several tissues in addition to skeletal muscle, including brain, heart, adipose tissue, kidneys and causes death about 3 weeks after birth. We analysed skeletal muscle and heart muscle tissues of Tk2 knockout mice at postnatal development phase and observed that TK2 deficient pups grew slower and their skeletal muscles appeared significantly underdeveloped, whereas heart was close to normal in size. Both tissues showed mtDNA depletion and mitochondria with altered ultrastructure, as revealed by transmission electron microscopy. Gene expression microarray analysis showed a strong down-regulation of genes involved in cell cycle and cell proliferation in both tissues, suggesting a lower pool of undifferentiated proliferating cells. Analysis of isolated primary myoblasts from Tk2 knockout mice showed slow proliferation, less ability to differentiate and signs of premature senescence, even in absence of mtDNA depletion. Our data demonstrate that TK2 deficiency disturbs myogenic progenitor cells function in postnatal skeletal muscle and we propose this as one of the causes of underdeveloped phenotype and myopathic characteristic of the TK2 deficient mice, in addition to the progressive mtDNA depletion, mitochondrial damage and respiratory chain deficiency in post-mitotic differentiated tissue.
Conflict of interest statement
Figures

References
-
- Berk AJ, Clayton DA (1973) A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid. J Biol Chem 248: 2722–2729. - PubMed
-
- Bogenhagen D, Clayton DA (1976) Thymidylate nucleotide supply for mitochondrial DNA synthesis in mouse L-cells. Effect of 5-fluorodeoxyuridine and methotrexate in thymidine kinase plus and thymidine kinase minus cells. J Biol Chem 251: 2938–2944. - PubMed
-
- Leanza L, Ferraro P, Reichard P, Bianchi V (2008) Metabolic interrelations within guanine deoxynucleotide pools for mitochondrial and nuclear DNA maintenance. J Biol Chem 283: 16437–16445. - PubMed
-
- Rampazzo C, Miazzi C, Franzolin E, Pontarin G, Ferraro P, et al. (2010) Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat Res 703: 2–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

