Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial
- PMID: 23341981
- PMCID: PMC3544843
- DOI: 10.1371/journal.pone.0053719
Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial
Abstract
Objective: Impaired liver regeneration is associated with a poor outcome in patients with decompensated alcoholic liver disease (ALD). We assessed whether autologous bone marrow mononuclear cell transplantation (BMMCT) improved liver function in decompensated ALD.
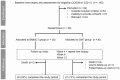
Design: 58 patients (mean age 54 yrs; mean MELD score 19, all with cirrhosis, 81% with alcoholic steatohepatitis at baseline liver biopsy) were randomized early after hospital admission to standard medical therapy (SMT) alone (n = 30), including steroids in patients with a Maddrey's score ≥32, or combined with G-CSF injections and autologous BMMCT into the hepatic artery (n = 28). Bone marrow cells were harvested, isolated and reinfused the same day. The primary endpoint was a ≥3 points decrease in the MELD score at 3 months, corresponding to a clinically relevant improvement in liver function. Liver biopsy was repeated at week 4 to assess changes in Ki67+/CK7+ hepatic progenitor cells (HPC) compartment.
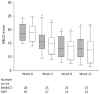
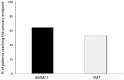
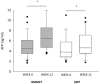
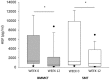
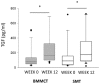
Results: Both study groups were comparable at baseline. After 3 months, 2 and 4 patients died in the BMMCT and SMT groups, respectively. Adverse events were equally distributed between groups. Moderate alcohol relapse occurred in 31% of patients. The MELD score improved in parallel in both groups during follow-up with 18 patients (64%) from the BMMCT group and 18 patients (53%) from the SMT group reaching the primary endpoint (p = 0.43 (OR 1.6, CI 0.49-5.4) in an intention to treat analysis. Comparing liver biopsy at 4 weeks to baseline, steatosis improved (p<0.001), and proliferating HPC tended to decrease in both groups (-35 and -33%, respectively).
Conclusion: Autologous BMMCT, compared to SMT is a safe procedure but did not result in an expanded HPC compartment or improved liver function. These data suggest either insufficient regenerative stimulation after BMMCT or resistance to liver regenerative drive in patients with decompensated alcoholic cirrhosis.
Trial registration: Controlled-Trials.com ISRCTN83972743.
Conflict of interest statement
Figures
References
-
- Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, et al. (2012) Acute-on chronic liver failure. J Hepatol 57(6): 1336–1348. - PubMed
-
- Mathurin P, Mendenhall CL, Carithers RL Jr, Ramond MJ, Maddrey WC, et al. (2002) Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol 36(4): 480–487. - PubMed
-
- EASL clinical practical guidelines (2012) management of alcoholic liver disease. J Hepatol 57(2): 399–420. - PubMed
-
- Ramond MJ, Poynard T, Rueff B, Mathurin P, Theodore C, et al. (1992) A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med 326(8): 507–512. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

