The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network
- PMID: 23341990
- PMCID: PMC3544906
- DOI: 10.1371/journal.pone.0053745
The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network
Abstract
Background: Ipilimumab, a cytotoxic T-lymphocyte antigen-4 (CTLA-4) blocking antibody, has been approved for the treatment of metastatic melanoma and induces adverse events (AE) in up to 64% of patients. Treatment algorithms for the management of common ipilimumab-induced AEs have lead to a reduction of morbidity, e.g. due to bowel perforations. However, the spectrum of less common AEs is expanding as ipilimumab is increasingly applied. Stringent recognition and management of AEs will reduce drug-induced morbidity and costs, and thus, positively impact the cost-benefit ratio of the drug. To facilitate timely identification and adequate management data on rare AEs were analyzed at 19 skin cancer centers.
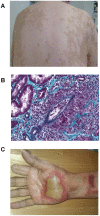
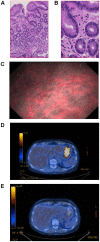
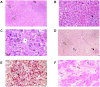
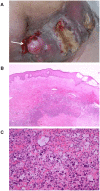
Methods and findings: Patient files (n = 752) were screened for rare ipilimumab-associated AEs. A total of 120 AEs, some of which were life-threatening or even fatal, were reported and summarized by organ system describing the most instructive cases in detail. Previously unreported AEs like drug rash with eosinophilia and systemic symptoms (DRESS), granulomatous inflammation of the central nervous system, and aseptic meningitis, were documented. Obstacles included patientś delay in reporting symptoms and the differentiation of steroid-induced from ipilimumab-induced AEs under steroid treatment. Importantly, response rate was high in this patient population with tumor regression in 30.9% and a tumor control rate of 61.8% in stage IV melanoma patients despite the fact that some patients received only two of four recommended ipilimumab infusions. This suggests that ipilimumab-induced antitumor responses can have an early onset and that severe autoimmune reactions may reflect overtreatment.
Conclusion: The wide spectrum of ipilimumab-induced AEs demands doctor and patient awareness to reduce morbidity and treatment costs and true ipilimumab success is dictated by both objective tumor responses and controlling severe side effects.
Conflict of interest statement
Figures
References
-
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364: 2517–2526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

