Probiotic bacteria induce a 'glow of health'
- PMID: 23342023
- PMCID: PMC3547054
- DOI: 10.1371/journal.pone.0053867
Probiotic bacteria induce a 'glow of health'
Abstract
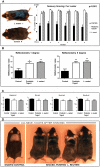
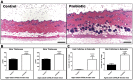
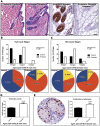
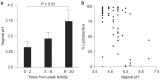
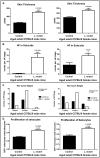
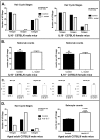
Radiant skin and hair are universally recognized as indications of good health. However, this 'glow of health' display remains poorly understood. We found that feeding of probiotic bacteria to aged mice induced integumentary changes mimicking peak health and reproductive fitness characteristic of much younger animals. Eating probiotic yogurt triggered epithelial follicular anagen-phase shift with sebocytogenesis resulting in thick lustrous fur due to a bacteria-triggered interleukin-10-dependent mechanism. Aged male animals eating probiotics exhibited increased subcuticular folliculogenesis, when compared with matched controls, yielding luxuriant fur only in probiotic-fed subjects. Female animals displayed probiotic-induced hyperacidity coinciding with shinier hair, a feature that also aligns with fertility in human females. Together these data provide insights into mammalian evolution and novel strategies for integumentary health.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

