A secretory protein of necrotrophic fungus Sclerotinia sclerotiorum that suppresses host resistance
- PMID: 23342034
- PMCID: PMC3544710
- DOI: 10.1371/journal.pone.0053901
A secretory protein of necrotrophic fungus Sclerotinia sclerotiorum that suppresses host resistance
Abstract
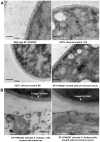
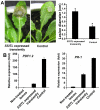
SSITL (SS1G_14133) of Sclerotinia sclerotiorum encodes a protein with 302 amino acid residues including a signal peptide, its secretion property was confirmed with immunolocalization and immunofluorescence techniques. SSITL was classified in the integrin alpha N-terminal domain superfamily, and its 3D structure is similar to those of human integrin α4-subunit and a fungal integrin-like protein. When S. sclerotiorum was inoculated to its host, high expression of SSITL was detected during the initial stages of infection (1.5-3.0 hpi). Targeted silencing of SSITL resulted in a significant reduction in virulence; on the other hand, inoculation of SSITL silenced transformant A10 initiated strong and rapid defense response in Arabidopsis, the highest expressions of defense genes PDF1.2 and PR-1 appeared at 3 hpi which was 9 hr earlier than that time when plants were inoculated with the wild-type strain of S. sclerotiorum. Systemic resistance induced by A10 was detected by analysis of the expression of PDF1.2 and PR-1, and confirmed following inoculation with Botrytis cinerea. A10 induced much larger lesions on Arabidopsis mutant ein2 and jar1, and slightly larger lesions on mutant pad4 and NahG in comparison with the wild-type plants. Furthermore, both transient and constitutive expression of SSITL in Arabidopsis suppressed the expression of PDF1.2 and led to be more susceptible to A10 and the wild-type strain of S. sclerotiorum and B. cinerea. Our results suggested that SSITL is an effector possibly and plays significant role in the suppression of jasmonic/ethylene (JA/ET) signal pathway mediated resistance at the early stage of infection.
Conflict of interest statement
Figures










Similar articles
-
The Arabidopsis Mediator Complex Subunit16 Is a Key Component of Basal Resistance against the Necrotrophic Fungal Pathogen Sclerotinia sclerotiorum.Plant Physiol. 2015 Sep;169(1):856-72. doi: 10.1104/pp.15.00351. Epub 2015 Jul 4. Plant Physiol. 2015. PMID: 26143252 Free PMC article.
-
An effector of a necrotrophic fungal pathogen targets the calcium-sensing receptor in chloroplasts to inhibit host resistance.Mol Plant Pathol. 2020 May;21(5):686-701. doi: 10.1111/mpp.12922. Epub 2020 Feb 27. Mol Plant Pathol. 2020. PMID: 32105402 Free PMC article.
-
A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum.New Phytol. 2018 Jan;217(2):739-755. doi: 10.1111/nph.14842. Epub 2017 Oct 27. New Phytol. 2018. PMID: 29076546
-
The evolutionary and molecular features of the broad-host-range plant pathogen Sclerotinia sclerotiorum.Mol Plant Pathol. 2022 Aug;23(8):1075-1090. doi: 10.1111/mpp.13221. Epub 2022 Apr 11. Mol Plant Pathol. 2022. PMID: 35411696 Free PMC article. Review.
-
Emerging Trends in Molecular Interactions between Plants and the Broad Host Range Fungal Pathogens Botrytis cinerea and Sclerotinia sclerotiorum.Front Plant Sci. 2016 Mar 31;7:422. doi: 10.3389/fpls.2016.00422. eCollection 2016. Front Plant Sci. 2016. PMID: 27066056 Free PMC article. Review.
Cited by
-
Alternative splicing reprogramming in fungal pathogen Sclerotinia sclerotiorum at different infection stages on Brassica napus.Front Plant Sci. 2022 Oct 12;13:1008665. doi: 10.3389/fpls.2022.1008665. eCollection 2022. Front Plant Sci. 2022. PMID: 36311105 Free PMC article.
-
De novo transcriptomic assembly and mRNA expression patterns of Botryosphaeria dothidea infection with mycoviruses chrysovirus 1 (BdCV1) and partitivirus 1 (BdPV1).Virol J. 2018 Aug 13;15(1):126. doi: 10.1186/s12985-018-1033-4. Virol J. 2018. PMID: 30103770 Free PMC article.
-
Root Transcriptome Analysis of Wild Peanut Reveals Candidate Genes for Nematode Resistance.PLoS One. 2015 Oct 21;10(10):e0140937. doi: 10.1371/journal.pone.0140937. eCollection 2015. PLoS One. 2015. PMID: 26488731 Free PMC article.
-
Jasmonate signaling and manipulation by pathogens and insects.J Exp Bot. 2017 Mar 1;68(6):1371-1385. doi: 10.1093/jxb/erw478. J Exp Bot. 2017. PMID: 28069779 Free PMC article. Review.
-
The complete genome sequence of the phytopathogenic fungus Sclerotinia sclerotiorum reveals insights into the genome architecture of broad host range pathogens.Genome Biol Evol. 2017 Mar;9(3):593-618. doi: 10.1093/gbe/evx030. Epub 2017 Feb 15. Genome Biol Evol. 2017. PMID: 28204478 Free PMC article.
References
-
- Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum . Can J Plant Pathol 16: 93–108.
-
- Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Path 7: 1–16. - PubMed
-
- Lai ZB, Wang F, Zheng ZY, Fan BF, Chen ZX (2011) A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. The Plant J 66: 953–968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

