Gene expression signatures that predict outcome of tamoxifen-treated estrogen receptor-positive, high-risk, primary breast cancer patients: a DBCG study
- PMID: 23342080
- PMCID: PMC3546921
- DOI: 10.1371/journal.pone.0054078
Gene expression signatures that predict outcome of tamoxifen-treated estrogen receptor-positive, high-risk, primary breast cancer patients: a DBCG study
Abstract
Background: Tamoxifen significantly improves outcome for estrogen receptor-positive (ER+) breast cancer, but the 15-year recurrence rate remains 30%. The aim of this study was to identify gene profiles that accurately predicted the outcome of ER+ breast cancer patients who received adjuvant Tamoxifen mono-therapy.
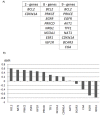
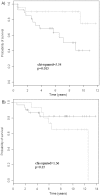
Methodology/principal findings: Post-menopausal breast cancer patients diagnosed no later than 2002, being ER+ as defined by >1% IHC staining and having a frozen tumor sample with >50% tumor content were included. Tumor samples from 108 patients treated with adjuvant Tamoxifen were analyzed for the expression of 59 genes using quantitative-PCR. End-point was clinically verified recurrence to distant organs or ipsilateral breast. Gene profiles were identified using a model building procedure based on conditional logistic regression and leave-one-out cross-validation, followed by a non-parametric bootstrap (1000x re-sampling). The optimal profiles were further examined in 5 previously-reported datasets containing similar patient populations that were either treated with Tamoxifen or left untreated (n = 623). Three gene signatures were identified, the strongest being a 2-gene combination of BCL2-CDKN1A, exhibiting an accuracy of 75% for prediction of outcome. Independent examination using 4 previously-reported microarray datasets of Tamoxifen-treated patient samples (n = 503) confirmed the potential of BCL2-CDKN1A. The predictive value was further determined by comparing the ability of the genes to predict recurrence in an additional, previously-published, cohort consisting of Tamoxifen-treated (n = 58, p = 0.015) and untreated patients (n = 62, p = 0.25).
Conclusions/significance: A novel gene expression signature predictive of outcome of Tamoxifen-treated patients was identified. The validation suggests that BCL2-CDKN1A exhibit promising predictive potential.
Conflict of interest statement
Figures

References
-
- Knoop AS, Bentzen SM, Nielsen MM, Rasmussen BB, Rose C (2001) Value of epidermal growth factor receptor, HER2, p53, and steroid receptors in predicting the efficacy of tamoxifen in high-risk postmenopausal breast cancer patients. J Clin Oncol 19: 3376–3384. - PubMed
-
- Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, et al. (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359: 2131–2139. - PubMed
-
- Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, et al. (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25: 486–492. - PubMed
-
- Mouridsen HT, Giobbie-Hurder A, Mauriac L, Paridaens R, Colleoni M, et al.. (2008) BIG 1–98: A randomized double-blind phase III study evaluating letrozole and tamoxifen given in sequence as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer. SABCS, abstract 13: webpage:abstracts2view.com/sabcs/view.php?nu = SABCS08L_553.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

