Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs
- PMID: 23342097
- PMCID: PMC3544809
- DOI: 10.1371/journal.pone.0054166
Validation of the zebrafish pentylenetetrazol seizure model: locomotor versus electrographic responses to antiepileptic drugs
Abstract
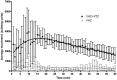
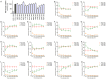
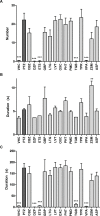
Zebrafish have recently emerged as an attractive in vivo model for epilepsy. Seven-day-old zebrafish larvae exposed to the GABA(A) antagonist pentylenetetrazol (PTZ) exhibit increased locomotor activity, seizure-like behavior, and epileptiform electrographic activity. A previous study showed that 12 out of 13 antiepileptic drugs (AEDs) suppressed PTZ-mediated increases in larval movement, indicating the potential utility of zebrafish as a high-throughput in vivo model for AED discovery. However, a question remained as to whether an AED-induced decrease in locomotion is truly indicative of anticonvulsant activity, as some drugs may impair larval movement through other mechanisms such as general toxicity or sedation. We therefore carried out a study in PTZ-treated zebrafish larvae, to directly compare the ability of AEDs to inhibit seizure-like behavioral manifestations with their capacity to suppress epileptiform electrographic activity. We re-tested the 13 AEDs of which 12 were previously reported to inhibit convulsions in the larval movement tracking assay, administering concentrations that did not, on their own, impair locomotion. In parallel, we carried out open-field recordings on larval brains after treatment with each AED. For the majority of AEDs we obtained the same response in both the behavioral and electrographic assays. Overall our data correlate well with those reported in the literature for acute rodent PTZ tests, indicating that the larval zebrafish brain is more discriminatory than previously thought in its response to AEDs with different modes of action. Our results underscore the validity of using the zebrafish larval locomotor assay as a rapid first-pass screening tool in assessing the anticonvulsant and/or proconvulsant activity of compounds, but also highlight the importance of performing adequate validation when using in vivo models.
Conflict of interest statement
Figures



References
-
- Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, et al. (2011) Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 52: 2–26. - PubMed
-
- Romanelli P, Striano P, Barbarisi M, Coppola G, Anschel DJ (2012) Non-resective surgery and radiosurgery for treatment of drug-resistant epilepsy. Epilepsy research. doi: 10.1016/j.eplepsyres.2011.12.016. - PubMed
-
- Löscher W (2011) Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 20: 359–368. - PubMed
-
- Watanabe Y, Takechi K, Fujiwara A, Kamei C (2010) Effects of antiepileptics on behavioral and electroencephalographic seizure induced by pentetrazol in mice. Journal of Pharmacological Sciences 112: 282–289. - PubMed
-
- Baraban SC, Taylor MR, Castro PA, Baier H (2005) Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131: 759–768. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

