Prospects for treating osteoarthritis: enzyme-protein interactions regulating matrix metalloproteinase activity
- PMID: 23342237
- PMCID: PMC3539270
- DOI: 10.1177/2040622312454157
Prospects for treating osteoarthritis: enzyme-protein interactions regulating matrix metalloproteinase activity
Abstract
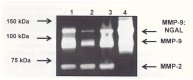
Primary osteoarthritis (OA) is a musculoskeletal disorder of unknown etiology. OA is characterized by an imbalance between anabolism and catabolism in, and altered homeostasis of articular cartilage. Matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motif are upregulated in OA joints. Extracellular matrix (ECM) proteins are critical for resistance to compressive forces and for maintaining the tensile properties of the tissue. Tissue inhibitor of metalloproteinases (TIMPs) is the endogenous inhibitor of MMPs, but in OA, TIMPs do not effectively neutralize MMP activity. Upregulation of MMP gene expression occurs in OA in a milieu of proinflammatory cytokines such as interleukin (IL)-1, IL-6 and tumor necrosis factor α. Presently, the medical therapy of OA includes mainly nonsteroidal anti-inflammatory drugs and corticosteroids which dampen pain and inflammation but appear to have little effect on restoring joint function. Experimental interventions to restore the imbalance between anabolism and catabolism include small molecule inhibitors of MMP subtypes or inhibitors of the interaction between IL-1 and its receptor. Although these agents have some positive effects on reducing MMP subtype activity they have little efficacy at the clinical level. MMP-9 is one MMP subtype implicated in the degradation of articular cartilage ECM proteins. MMP-9 was found in OA synovial fluid as a complex with neutrophil gelatinase-associated lipocalin (NGAL) which protected MMP-9 from autodegradation. Suppressing NGAL synthesis or promoting NGAL degradation may result in reducing the activity of MMP-9. We also propose initiating a search for enzyme-protein interactions to dampen other MMP subtype activity which could suppress ECM protein breakdown.
Keywords: gelatinase; matrix metalloproteinase; neutrophil gelatinase-associated lipocalin; osteoarthritis.
Conflict of interest statement
Figures

References
-
- Aida Y., Maeno M., Suzuki N., Shiratsuchi H., Motohashi M, Matsumura H. (2005) The effect of IL-1β on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci 77: 3210–3221 - PubMed
-
- Ala-aho R., Kahari V. (2005) Collagenases and cancer. Biochimie 87: 273–286 - PubMed
-
- Alcaraz M., Megías J., García-Arnandis I., Clérigues V., Guillén M. (2010) New molecular targets for the treatment of osteoarthritis. Biochem Pharmacol 80: 13–21 - PubMed
-
- Bergmann U., Tuuttila A., Stetler-Stevenson W., Tryggvason K. (1995) Autolytic activation of recombinant human 72 kilodalton type IV collagenase. Biochemistry 34: 2819–2825 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

