ERBB3 (HER3) is a key sensor in the regulation of ERBB-mediated signaling in both low and high ERBB2 (HER2) expressing cancer cells
- PMID: 23342251
- PMCID: PMC3544427
- DOI: 10.1002/cam4.10
ERBB3 (HER3) is a key sensor in the regulation of ERBB-mediated signaling in both low and high ERBB2 (HER2) expressing cancer cells
Abstract
Aberrant expression and activation of EGFR and ERBB2 (HER2) have been successfully targeted for cancer therapeutics. Recent evidence from both basic and clinical studies suggests that ERBB3 (HER3) serves as a key activator of downstream signaling through dimerization with other ERBB proteins and plays a critical role in the widespread clinical resistance to EGFR and HER2 targeting cancer therapies. As a result, HER3 is actively pursued as an antibody therapeutic target for cancer. Ligand binding is thought to be a prerequisite for dimerization of HER3 with other ERBB proteins, which results in phosphorylation of its c-terminal tyrosine residues and activation of downstream AKT and MAPK signaling pathways. In this study, we report that an anti-HER2 monoclonal antibody (HER2Mab), which blocks HER2 dimerization with HER3, induces HER3 dimerization with EGFR in both low and high HER2 expressing cancer cells. Treatment of the low HER2 expressing MCF7 cancer cells with HER2Mab promoted cell proliferation and migration in the absence of HER3 ligand stimulation. Follow-up studies revealed that HER2Mab-induced HER3 signaling via EGFR/HER3 dimerization and activation of downstream AKT signaling pathways. These results suggest that equilibrium of dimerization among the ERBB proteins can be perturbed by HER2Mab and HER3 plays a key role in sensing the perturbation.
Keywords: Anti-HER2 antibody; EGFR; ERBB2/HER2; ERBB3/HER3; MCF7; signaling.
Figures

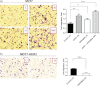
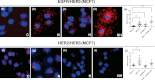


References
-
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5:341–354. - PubMed
-
- Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci. Signal. 2009;2:ra31. - PubMed
-
- Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer. 2009;9:463–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

