Triple-negative breast cancer: multipronged approach, single-arm pilot phase II study
- PMID: 23342258
- PMCID: PMC3544434
- DOI: 10.1002/cam4.3
Triple-negative breast cancer: multipronged approach, single-arm pilot phase II study
Abstract
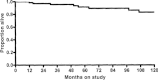
Anthracyclines (A) and taxanes (T) are standard first-line chemotherapy agents for patients with advanced breast cancer. Platinum analogues have also shown activity in the triple-negative breast cancer (TNBC) histology, but clinical data are limited. Here we report the long-term follow-up of a phase II study on TNBC treated with a combined modality therapy, including induction with AT, cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) with concurrent radiation therapy, and a dose-dense consolidation chemotherapy (HDCT) with carboplatin (CBDCA), ifosfamide (IFX), etoposide (VP-16). Patients' median age was 44 years, with 73% premenopausal. Epirubicin 75 mg/m(2) and docetaxel 75 mg/m(2) were administered to 70 patients with TNBC: as neoadjuvant and adjuvant therapy to 12 and 58 patients, respectively. Postoperative radiation therapy, 5000 cGy, was delivered, synchronous with triweekly CMF. After radiation therapy, two courses of HDCT with CBDCA, IFX, VP-16, were given, with hematological growth factors. After a median follow-up of 81 months, all patients were evaluable for toxicity and response. Most important toxicity were grade 3 skin reaction and grade 4 hematological in 3% and 31% of patients, respectively. Pathological complete response was observed in 25% of patients receiving preoperative chemotherapy. Treatment failures were as follows: eight visceral, four contralateral breast cancer, four locoregional, and one leukemia. Five-year progression-free survival and overall survival rate were 78% and 91%, respectively. Induction chemotherapy, followed by chemoradiation therapy and HDCT, provides a prolonged disease-free period and a significant increase in overall survival in TNBC, with an acceptable toxicity profile.
Keywords: Concurrent chemotherapy and radiation therapy; high-dose chemotherapy; platinum analogues; triple-negative breast cancer.
Figures

Similar articles
-
Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP).Clin Cancer Res. 2021 Feb 15;27(4):975-982. doi: 10.1158/1078-0432.CCR-20-3646. Epub 2020 Nov 18. Clin Cancer Res. 2021. PMID: 33208340 Free PMC article. Clinical Trial.
-
Carboplatin plus taxanes are non-inferior to epirubicin plus cyclophosphamide followed by taxanes as adjuvant chemotherapy for early triple-negative breast cancer.Breast Cancer Res Treat. 2020 Jul;182(1):67-77. doi: 10.1007/s10549-020-05648-9. Epub 2020 May 11. Breast Cancer Res Treat. 2020. PMID: 32394350 Clinical Trial.
-
Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact.Ann Oncol. 2014 Aug;25(8):1570-7. doi: 10.1093/annonc/mdu183. Epub 2014 May 14. Ann Oncol. 2014. PMID: 24827135 Clinical Trial.
-
Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer.Cochrane Database Syst Rev. 2019 Feb 18;2(2):CD012873. doi: 10.1002/14651858.CD012873.pub2. Cochrane Database Syst Rev. 2019. PMID: 30776132 Free PMC article.
-
Current strategy for triple-negative breast cancer: appropriate combination of surgery, radiation, and chemotherapy.Breast Cancer. 2011 Jul;18(3):165-73. doi: 10.1007/s12282-011-0254-9. Epub 2011 Feb 3. Breast Cancer. 2011. PMID: 21290263 Review.
Cited by
-
miRNA - Therapeutic tool in breast cancer? Where are we now?Rep Pract Oncol Radiother. 2014 Nov 26;20(2):79-86. doi: 10.1016/j.rpor.2014.10.009. eCollection 2015 Mar-Apr. Rep Pract Oncol Radiother. 2014. PMID: 25859396 Free PMC article. Review.
-
Clinical Efficacy and Quality of Life Assessment of Partial Cystectomy and Plasmakinetic Transurethral Resection of Tumor in Bladder Cancer Patients.Cancer Manag Res. 2022 Jan 28;14:389-398. doi: 10.2147/CMAR.S346764. eCollection 2022. Cancer Manag Res. 2022. PMID: 35115835 Free PMC article.
-
Anthracycline-based induction chemotherapy followed by concurrent cyclophosphamide, methotrexate and 5-fluorouracil and radiation therapy in surgically resected axillary node-positive breast cancer.Mol Clin Oncol. 2014 May;2(3):473-478. doi: 10.3892/mco.2014.269. Epub 2014 Mar 21. Mol Clin Oncol. 2014. PMID: 24772320 Free PMC article.
References
-
- Dent R, Trudeau M, Pritchard KI, et al. Triplenegative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13:4429–4434. - PubMed
-
- Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

