Biomarker-driven trial in metastatic pancreas cancer: feasibility in a multicenter study of saracatinib, an oral Src inhibitor, in previously treated pancreatic cancer
- PMID: 23342270
- PMCID: PMC3544442
- DOI: 10.1002/cam4.27
Biomarker-driven trial in metastatic pancreas cancer: feasibility in a multicenter study of saracatinib, an oral Src inhibitor, in previously treated pancreatic cancer
Abstract
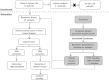
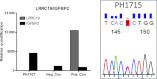
Src tyrosine kinases are overexpressed in pancreatic cancers, and the oral Src inhibitor saracatinib has shown antitumor activity in preclinical models of pancreas cancer. We performed a CTEP-sponsored Phase II clinical trial of saracatinib in previously treated pancreas cancer patients, with a primary endpoint of 6-month survival. A Simon MinMax two-stage phase II design was used. Saracatinib (175 mg/day) was administered orally continuously in 28-day cycles. In the unselected portion of the study, 18 patients were evaluable. Only two (11%) patients survived for at least 6 months, and three 6-month survivors were required to move to second stage of study as originally designed. The study was amended as a biomarker-driven trial (leucine rich repeat containing protein 19 [LRRC19] > insulin-like growth factor-binding protein 2 [IGFBP2] "top scoring pairs" polymerase chain reaction [PCR] assay, and PIK3CA mutant) based on preclinical data in a human pancreas tumor explant model. In the biomarker study, archival tumor tissue or fresh tumor biopsies were tested. Biomarker-positive patients were eligible for the study. Only one patient was PIK3CA mutant in a 3' untranslated region (UTR) portion of the gene. This patient was enrolled in the study and failed to meet the 6-month survival endpoint. As the frequency of biomarker-positive patients was very low (<3%), the study was closed. Although we were unable to conclude whether enriching for a subset of second/third line pancreatic cancer patients treated with a Src inhibitor based on a biomarker would improve 6-month survival, we demonstrate that testing pancreatic tumor samples for a biomarker-driven, multicenter study in metastatic pancreas cancer is feasible.
Keywords: Biomarker; Src; clinical trial; pancreas cancer.
Figures

Similar articles
-
Common PIK3CA mutants and a novel 3' UTR mutation are associated with increased sensitivity to saracatinib.Clin Cancer Res. 2012 May 1;18(9):2704-14. doi: 10.1158/1078-0432.CCR-11-3167. Clin Cancer Res. 2012. PMID: 22553375 Free PMC article.
-
A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer.Invest New Drugs. 2012 Apr;30(2):779-86. doi: 10.1007/s10637-010-9611-3. Epub 2010 Dec 18. Invest New Drugs. 2012. PMID: 21170669 Clinical Trial.
-
A phase II study of saracatinib (AZD0530), a Src inhibitor, administered orally daily to patients with advanced thymic malignancies.Lung Cancer. 2015 Jul;89(1):57-60. doi: 10.1016/j.lungcan.2015.04.008. Epub 2015 Apr 25. Lung Cancer. 2015. PMID: 26009269 Clinical Trial.
-
Current status of SRC inhibitors in solid tumor malignancies.Oncologist. 2011;16(5):566-78. doi: 10.1634/theoncologist.2010-0408. Epub 2011 Apr 26. Oncologist. 2011. PMID: 21521831 Free PMC article. Review.
-
Dual Drug Repurposing: The Example of Saracatinib.Int J Mol Sci. 2024 Apr 22;25(8):4565. doi: 10.3390/ijms25084565. Int J Mol Sci. 2024. PMID: 38674150 Free PMC article. Review.
Cited by
-
Current status and dilemma of second-line treatment in advanced pancreatic cancer: is there a silver lining?Onco Targets Ther. 2018 Aug 6;11:4591-4608. doi: 10.2147/OTT.S166405. eCollection 2018. Onco Targets Ther. 2018. PMID: 30122951 Free PMC article. Review.
-
LRRC19-A Bridge between Selenium Adjuvant Therapy and Renal Clear Cell Carcinoma: A Study Based on Datamining.Genes (Basel). 2020 Apr 17;11(4):440. doi: 10.3390/genes11040440. Genes (Basel). 2020. PMID: 32316597 Free PMC article.
-
The NIH-Industry New Therapeutic Uses Pilot Program: Demonstrating the Power of Crowdsourcing.Assay Drug Dev Technol. 2015 Jul-Aug;13(6):297-8. doi: 10.1089/adt.2015.29006.cmcdrrr. Assay Drug Dev Technol. 2015. PMID: 26241208 Free PMC article. No abstract available.
-
Phase II clinical trials on investigational drugs for the treatment of pancreatic cancers.Expert Opin Investig Drugs. 2015 Jun;24(6):781-94. doi: 10.1517/13543784.2015.1026963. Epub 2015 Mar 25. Expert Opin Investig Drugs. 2015. PMID: 25809274 Free PMC article. Review.
-
Identification of lymphocyte cell-specific protein-tyrosine kinase (LCK) as a driver for invasion and migration of oral cancer by tumor heterogeneity exploitation.Mol Cancer. 2021 Jun 11;20(1):88. doi: 10.1186/s12943-021-01384-w. Mol Cancer. 2021. PMID: 34116687 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61:69–90. - PubMed
-
- Yeo TP, Hruban RH, Leach SD, et al. Pancreatic cancer. Current Problems in Cancer. 2002;26:176–275. - PubMed
-
- Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology. 1997;15:2403–2413. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England Journal of Medicine. 2011;364:1817–1825. - PubMed
-
- Irby R, Yeatman T. Role of Src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

